return to: Pediatric Airway or Change of Tracheostomy Tube
General Considerations
-
Protracted tracheostomy can lead to numerous medical, social, and economic consequences in pediatric patients
-
Tracheal infections and stenosis
-
Speech delays
-
Social stigma and life-style changes
-
Financial burden
-
-
Decannulation protocols vary by institution and surgeon.
-
Numerous decannulation techniques have been reported, however, many are overly intensive, costly, and require repetitive studies/interventions
-
Some decannulation protocols may involve sleep studies, repetitive laser procedures, and tracheostomy tube "downsizing" over an extended period of time (Waddell et al. 1997; Gray et al. 1998). Conversely, some advocate simple decannulation followed by only overnight monitoring (Wirtz et al. 2016).
-
The common theme for all protocols is that a direct laryngoscopy/bronchoscopy is required to assess the airway prior to proceeding with decannulation protocol.
-
-
For pediatric patients aged 5 and under, we recommend a decannulation protocol that requires creation of a fenestrated tracheostomy (Merritt et al. 1997).
-
Children under the age of 5 have small caliber airways, such that even the smallest tracheostomy tubes may occupy the entire tracheal lumen. A corked tube in such an airway would occlude the entire airway, resulting in a decannulation trial failure in a child that may have otherwise done well in the event of actual decannulation. For this reason, we consider a simple corking trial to be an unreliable indicator of a small child's readiness for decannulation.
-
A successful 12 to 24-hour trial with a fenestrated, corked tracheotosmy with appropriate oxygen saturation allows for a safe and timely decanulation process without undue hospital stay.
-
A fenestrated, corked tracheostomy trial simulates a small child's ability to maintain their own airway through the fenestration yet still allows for rapid intervention should airway compromise occur.
-
-
A 2013 consensus statement from the American Academy of Otolaryngology (Mitchell et al. 2013) indicated the following criteria should be met prior to proceeding with pediatric tracheostomy decannulation:
-
No ventilatory support required for 3 months prior to decannulation (time may range from 2-4 months to account for winter versus summer months)
-
No aspiration events should occur in the patient that would preclude decannulation
-
Flexible laryngoscopy should show patent airway with at least one mobile vocal cord
-
Suprastomal granulation tissue should be excised prior to decannulation
-
Operative Procedure
-
Pediatric direct laryngoscopy should be performed to assess the airway. See Pediatric Direct Laryngoscopy for details.
-
Any suprastomal granulation tissue should be removed -- we prefer curved upbiting Kerrison rongeurs. All granulation tissue, regardless of size, should be removed prior to decannulation process.
-
A fenestrated tracheostomy tube is then fashioned.
-
The tracheostomy tube used is typically one size smaller than the current tube.
-
Hash marks are drawn on the tracheostomy tube's upper surface and the tracheostomy tube is placed in the airway and visualized by endoscopy. This allows precise cutting of the fenestration once the tracheostomy tube is removed.
-
The tracheostomy tube is removed and the fenestration is cut to size using an 11 blade.
-
The adequacy of the fenestration is confirmed via endoscopy.
-
-
At the end of the procedure, the patient's regular tracheostomy tube is placed back in the airway.
-
The patient is admitted for overnight observation on continuous pulse oximetry.
-
The next morning the fenestrated tracheostomy is then placed and immediately capped.
-
The patient is observed for 24 hours.
-
Adequate oxygenation is considered to be > 90%
-
The observation period should include overnight sleep and a session of routine physical activity.
-
This is crucial in identifying potential precipitating factors leading to desaturation during activities outside of the hospital
-
A successful trial should be free of episodes of cyanosis, retractions, or tachypnea.
-
The child should not be sedated during the initiation of the trial due to the increase in false positives for airway compromise
-
-
After 24 hours of observation with the capped, fenestrated tracheostomy tube, the fenestrated tracheostomy tube is then removed and the tracheostomy is allowed to heal by secondary intention.
-
The patient may be observed for 12-24 hours if desired.
-
The tracheostomy site should not be covered with an occlusive dressing that traps air in the neck.
-
-
In the event of decannulation trial failure, the fenestrated tracheostomy tube must be removed and the child's nonfenestrated tube replaced prior to discharge from the hospital.
-
Fenestrated tracheostomy tubes carry a well-documented risk of granulation tissue formation if left in place for a long period of time (Carron et al. 2006). This complication has never been observed at the University of Iowa since implementation of this protocol in the early 1980s, since the fenestrated tube is never in place for longer than 48 hours.
-
It is our practice to store the fenestrated trach within the pediatric otolaryngology department when not in use; the fenestrated tube is never sent home with the patient to avoid inappropriate long-term use of the fenestrated tracheotomy tube at home.
-
Parents are also educated as to why the fenestrated tracheostomy tube must not be left in place beyond the decannulation trial hospital stay.
-
Dictation template
Informed consent was reviewed with the parents. After induction and mask ventilation by anesthesia staff, a time-out was performed. The bed was turned 90 degrees from anesthesia. At this time the airway was handed over to the otolaryngology team. A Miller 1 blade was used to visualize the glottis - a grade 1 view with cricoid pressure was achieved and 1cc of preservative free lidocaine was applied to the cords. Mask ventilation was resumed briefly until oxygen saturation reached 100%. Next, the maxillary dentition was protected and the infant Lindholm was placed into the vallecula and placed into suspension. The anesthesia circuit was connected directly to the laryngoscope. Photodocumentation was obtained with the zero degree rigid endoscope.
There was a small amount of suprastomal granulation tissue which was removed using upbiting curved Kerrison rongeurs through the stoma under direct visualization through the glottis with the zero degree rigid endoscope. Hemostasis was obtained with afrin soaked pledgets. There was no tracheomalacia and bilateral mainstem bronchi were visualized with no bronchomalacia. There was follicular bronchitis. Palpation of the supraglottis showed no fixation. The airway was sized to 4.5 ETT with a large leak but some resistance at the stoma. We did not see evidence of subglottic stenosis. We then fashioned a 3.5 uncuffed shiley trach tube with a fenestration directly in the airway. This was saved for use during decannulation. The patient's original trach was replaced without difficulty.
The laryngoscope was removed and the airway handed back to anesthesia. The patient tolerated the procedure well.
Images
1. Endoscopic (direct laryngoscopy) view of subglottis and upper trachea with suprastomal granulation tissue

2. Suprastomal granulation tissue
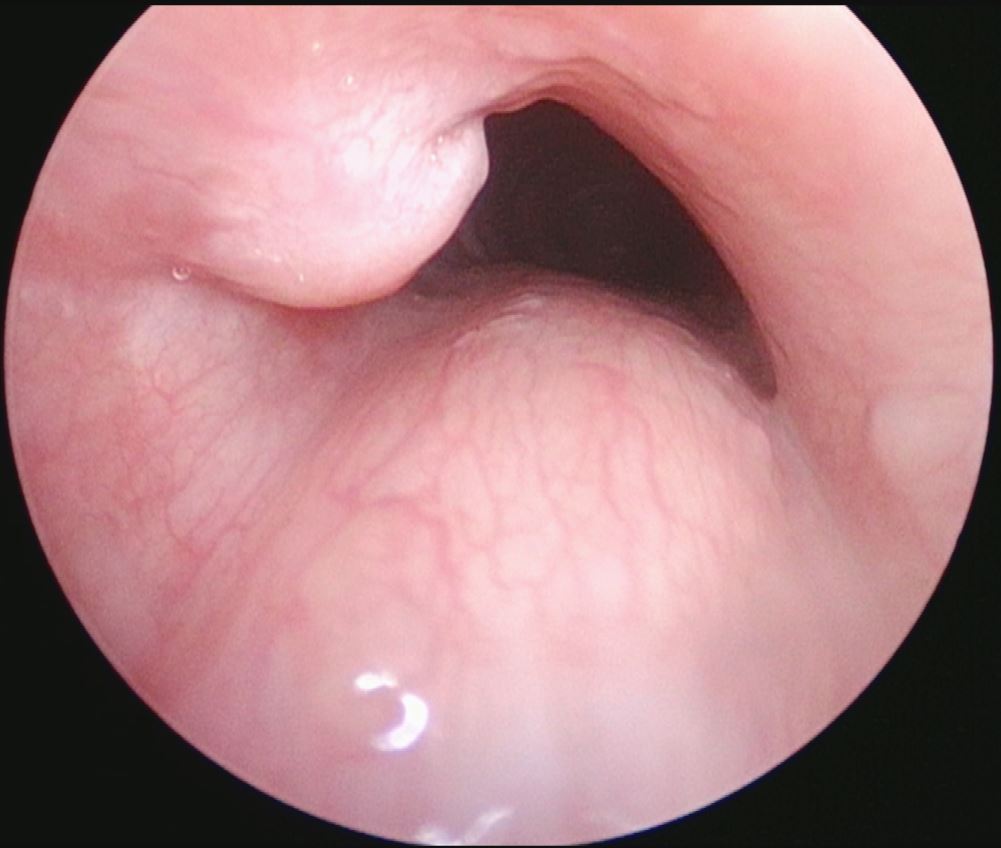
3. Suprastomal granulation tissue being removed with Kerrison rongeur placed through tracheostome
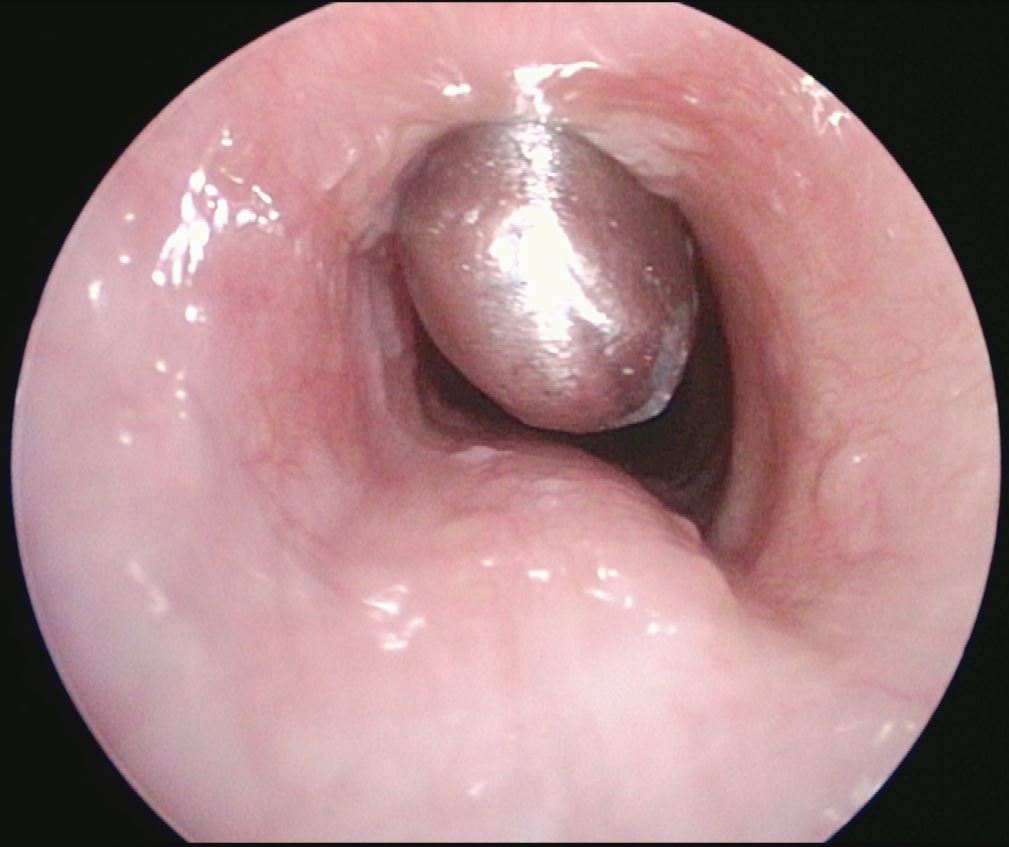
4. After removal of suprastomal granulation tissue now with fenestrated tracheotomy tube in place.
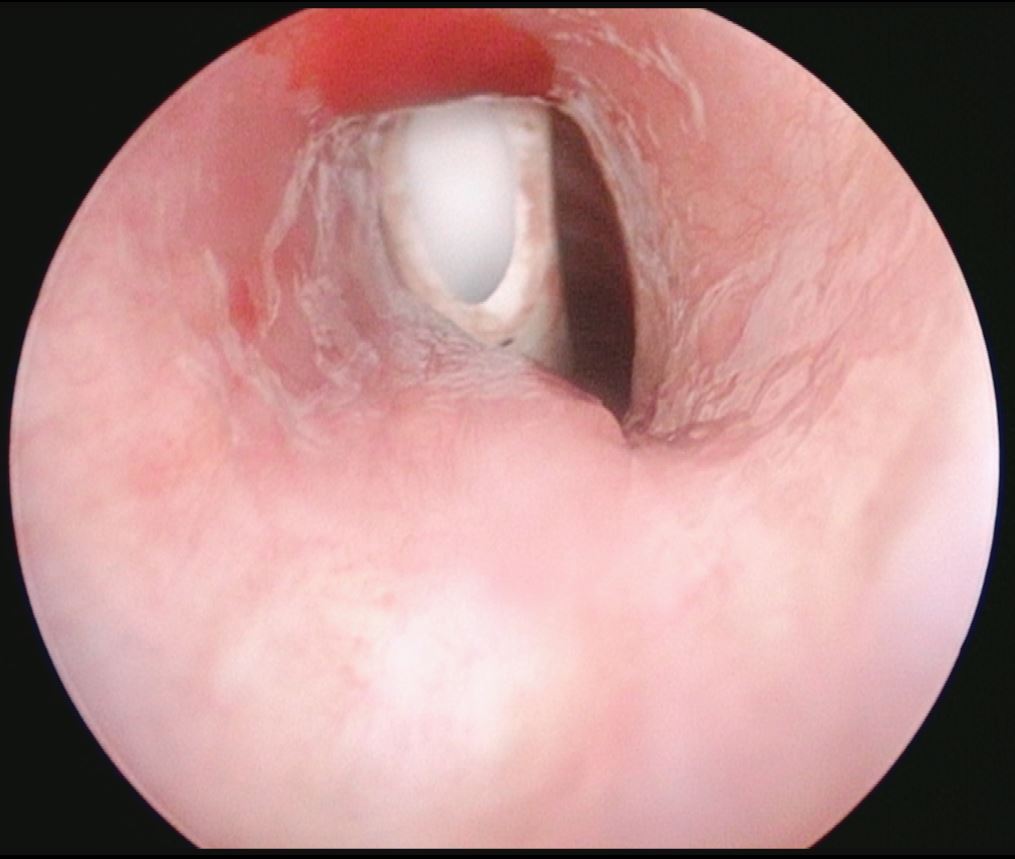
References
Waddell, A., et al. "The Great Ormond Street protocol for ward decannulation of children with tracheostomy: increasing safety and decreasing cost." International journal of pediatric otorhinolaryngology 39.2 (1997): 111-118.
Gray, Robert F., N. Wendell Todd, and Ian N. Jacobs. "Tracheostomy decannulation in children: approaches and techniques." The Laryngoscope 108.1 (1998): 8-12.
Wirtz, Nicholas, et al. "A pediatric decannulation protocol: outcomes of a 10-year experience." Otolaryngology--Head and Neck Surgery 154.4 (2016): 731-734.
Merritt, Robert M., John P. Bent, and Richard JH Smith. "Suprastomal granulation tissue and pediatric tracheotomy decannulation." The Laryngoscope 107.7 (1997): 868-871.
Mitchell, Ron B., et al. "Clinical consensus statement: tracheostomy care." Otolaryngology--Head and Neck Surgery148.1 (2013): 6-20.
Seligman KL, Liming BJ, Smith RJH. Pediatric Tracheostomy Decannulation: 11-Year Experience. Head Neck Surg. 2019 Sep;161(3):499-506. doi: 10.1177/0194599819842164. Epub 2019 Apr 16.
Carron MA, Kim SA, Sawhney R, Reidy P. "Airway obstruction by granulation tissue within a fenestrated tracheostomy tube: case report." Ear Nose Throat Journal 85 (2006): 54-55.



