return to: Botulinum neurotoxin treatment of salivary gland disorders; Botox injection to salivary glands for hypersalivation; Botulinum Toxin Treatment for Sialadenitis (OnabotulinumtoxinA - Botox®) (IncobotulinumA - Xeomin®) and (AbobotulinumtoxinA - Dysport®) Salivary Swelling; Case example EMG guided laryngeal Botox Injection; Case Example Vocal Tremor Response to Botox
Seven serotypes (BoNT/A to G) of the neurotoxin produced by the gram-positive bacteria Clostridium botulinum have been identified. The serotypes A and B are used clinically.
Mechanism of Action
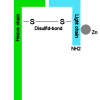
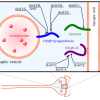
- Inhibition of release of the neurotransmitter acetylcholine at the neuro-effector junction. This botox effect results from inhibition of exocytotic mechanisms within the neurons - to temporarily inhibit acetylcholine release at the neuro-effector junction (Walker and Dayan, 2014). The active neurotoxin molecule is contained within a larger aggregate of clinically inactive proteins that stabilize the assembly from pH and temperature changes as well as enzymatic degradation. Each serotype of the neurotoxin has the same protein structure, but differs in protein sequences to account for different affinities, antigenicities and intracellular targets.
- The neurotoxin protein assembly binds to the nerve ending (synaptic vesicle) using its heavy chain and enters the neural cell by endocytosis. The acidic pH in the endocytotic vesicle cleaves the disulfide.
- In addition to inhibition of acetylcholine release, the degradation of function of SNARE proteins by botulinum toxin has other potential therapeutic implications. SNARE proteins play a role in the C-fiber nociceptive neurons that release glutamate and substance P - both of which result in vasodilation and release of pro-inflammatory mediators such as bradykinin, prostaglandins, histamine and serotonin. This mechanism of action may account for the successful use of botulinum toxin in the management of chronic pain disorders such as migraine headaches (Walker and Dayan, 2014). Botulinum toxins appear to have a significantly longer effect on autonomic neurons (6-9 months) than for striated muscle (3-4 months) - interpreted to identify that neuronal sprouting does not occur to the same degree for autonomic nervous system as for striated muscle.
onabotulinumtoxinA (onBoNT-A) = Botox ®/Botox Cosmetic® (Allergan, Inc). The original botulinum toxin type A product; a formulation change occurred in 1997 to reduce the amount of immunogenic protein content. (Botox® comprises 85% of the worldwide Botox® market)
incobotulinumtoxinA (incoBoNT-A) = Xeomin® (Merz Pharmaceuticals). Approved in 2011 by the FDA for temporary improvement in the appearance of moderate-to-severe glabellar lines in adults. IncobotulinumtoxinA (Xeomin®) is prepared by stripping away complexing proteins and thought to be a purer formulation than onabotulinumtoxinA (Botox®) that could lead to greater efficacy with reduced sensitization to antibody formation. Walker and Dayan (2014) relate the clinical relevance of this potential improvement has yet to be determined. Xeomin has been reported to have a dose ration with botox of 1:1 (Carruthers and Carruthers, 2007)
abobotulinumtoxinA (aboBoNT-A) = Dysport® (Medicis Pharmaceutical Corp.). Has the same active neurotoxin (botulinum toxin type A) but differs from onabotluinumtoxinA by its method of purification. Dysport® is thought to have a more diffuse distribution of effect (greater spread than Botox®). Dose ratios have been debated, with the most commonly agreed dose ration of ~ 3:1 (Kranz et al. 2009).
rimabotulinumtoxinB = Myobloc® (Solstice Neurosciences, Inc.). The only botulinum toxin type B commercially available in the U.S. More rapid onset of action and greater area of diffusion are reported at the expense of more painful injections and shorter duration of effects. Dose ratio for equivalent effects to Botox® have been reported to be 50:1 for cervical dystonia and 100:1 for glabellar frown lines (Spencer et al. 2002). Neurobloc® (Eisai Co.,Ltd) is the European equivalent to Myobloc®.
Immunogenicity from Botulinum Toxins
- Addressed by Carr et al. (2021): These investigators identify switching from the more immunogenic preparation (onaBoNT-A or aboBoNT-A) to a less immunogenic second-generation BoNT-A formulation with lower potential immunogenicity (incoBoNT-A) is reasonable - especially when signs of clinical resistance develop. Increased signs of clinical resistance include need for increased dosage and shortened injection intervals - with this change to a less immunogenic preparation "ideally occur early enough to prove effective in restoring an optimal clincal response"
Rash Reported in Association with Botulinum Toxins
- Blumenfeld et al. (2018) reported a prospective long-term study of 716 adults receiving onabotulinum toxin injections to 7 head/neck muscles every 12 weeks (+/- 7 days) over 108 weeks. Treatment related adverse events were reported by 60.0% in the course of the study with 4.5% discontinuing treatment due to treatment associated effects. Neck pain (4.1%), eyelid ptosis (2.5%), musculoskeletal stiffness (2.4%), injection site pain (2.0%), headache (1.7%), muscular weakness (1.4%), and facial paresis (1.3%) were among the most common. Three patients (0.4%) reported a rash sufficient to discontinue treatment - one patient of which was considered 'serious treatment-related adverse event' (rash). No images or further description of the appearance of the rash was provided in their publication.
- As per Arbizu et al. (2015) "use of BT for gastroenterological conditions, there are significant complications ranging from minor pain, rash and allergic reactions"
- Mezaki and Sakai (2005) reported development of "a miliary skin rash ...on her body trunk, arms, and lower limbs with an itchy sensation" approximately 30 hours after receiving botulinum toxin type A (total dose 24 units) to treat blepharospasm. The skin rash was depicted with an image and described as "erythematous macules and papules"- with a biopsy consistent with the histology of drug eruption. Treatment of 'epinastine hydrochlroide' was initiated and the rash disappeared after 2 weeks - with no further treatment with botulinum toxin injections.
- Brüggemann et al. (2009) reported two cases of rash developing one hour and six hours after administration of DYSPORT (R) with one rash reported as resolving without therapy over a 1 day course - followed by evaluation by "Intracutaneous and prick tests for both BOTOX(R) and DYSPORT (R) were unremarkable - retreat was done with DYSPORT 3 months later and 'tolerated re-exposure without relapse".
Botulinum toxin use during pregnancy
Li and Tang (2020) through assessment of current literature concluded:
- "BoNT [botulinum toxin] does not increase the risk of complication in pregnant women and fetuses", however-
- "use of BoNT to treat disease during pregnancy requires fully informed consent from patients"
- "further research is needed to determine how to rescued the side effects of BoNT injection during pregnancy"
Brin et al. (2016) through review of 'the Allergan Global Safety Database' containing reports of onabotulinumtoxinA administration before/during pregnancy (search from 1-1-90 to 12-31-2013) wherein treatment occurred during pregnancy or ≤ 3 months before conception.
- As a preamble to their study - they cited US Department of Health and Human Services 2013: "OnabotulinumtoxinA is designated as a US Food and Drug Administration (FDA) pregnancy category C pharmaceutical product, as there are no adequate and well controlled studies in pregnant women, and it should only be used during pregnancy if the benefits outweigh the potential risks."
- Also cited data from Tang-Liu et al. (2003) and Ravichandran et al. (2006) in offering the statement that "OnabotulinumtoxinA is not expected to be present in peripheral blood at measurable levels following intramuscular or intradermal injection at the recommended doses, and preclinical studies predict that any potentially systemic onabotulinumtoxinA would be completely eliminated from the systemic circulation within 48 h post-injection in rodents"
Brin et al. study (2016):
- 574 pregnancies identified among which 232 were eligible with known outcomes
- 137 with record of dose identified <50 U (40.1%); 50 U to <100 U (14.6%); 100 U to <200 U (27.7%); >200U (17.5%)
- Most (96.0%) fetal exposures occurred during/before the first trimester
- Of the 137 prospective cases (139 fetuses):
- 110 (79.1%) were live births
- 29 (20.9%) ended in fetal loss (21 spontaneous, 8 induced abortions)
- Among live births - 106 (96.4%) were normal with four abnormal birth outcomes (1 major fetal defect, 2 minor fetal malformations, 1 birth complication) = 2.7% prevalence rate for overall fetal defects
- Of the 137 prospective cases (139 fetuses):
- Concluding with this 24 year retrospective review showed 'prevalence of fetal defects in onabotulinumtoxinA-exposed mothers before/during pregnancy (2.7%) is comparable with background rates in the general population
References
US Department of Health and Human Services. Title 21-Food and Drugs. Chapter I-Food and Drug Administration, Department of Health and Human Services. Subchapter C-Drugs: General. Part 201-Labeling. Rockville, MD: FDA, 2013
Tang-Liu DD, Aoki KR, Dolly JO, et al. Intramuscular injection of 125I-botulinum neurotoxin-complex versus 125I-botulinum-free neurotoxin: time course of tissue distribution. Toxicon 2003; 42: 461–469.
Ravichandran E, Gong Y, Al Saleem FH, Ancharski DM, Joshi SG, Simpson LL. An initial assessment of the systemic pharmacokinetics of botulinum toxin. J Pharmacol Exp Ther 2006; 318: 1343–1351
Li W, Tang M. Application of botulinum toxin in pregnancy and its impact on female reproductive health. Expert Opin Drug Saf. 2020 Jan;19(1):83-91. doi: 10.1080/14740338.2020.1707803. Epub 2019 Dec 26. PMID: 31868020.
Brin MF, Kirby RS, Slavotinek A, Miller-Messana MA, Parker L, Yushmanova I, Yang H. Pregnancy outcomes following exposure to onabotulinumtoxinA. Pharmacoepidemiol Drug Saf. 2016 Feb;25(2):179-87. doi: 10.1002/pds.3920. Epub 2015 Dec 4. PMID: 26635276; PMCID: PMC5063122.
Carruthers J and Carruthers A: The evolution of botulinum neurotoxin type A for cosmetic applications. J Socmet Laser Ther. 2007;9(3):186-192
Kranz G, Haubenberger D, Voller B, et al Respective potencies of Botox® and Dysport® in a human skin model: randomized double-blind study. Mov Disord. 2009;24:231-236
Spencer JM, Gordon M, Goldberg DJ. Botulinum B treatment of the glabellar and frontalis regions: a dose repsonse analysis. J Cosmet Laser Ther. 2002;4:19-23
Walker TJ, Dayan SH: Comparison and Overview of Currently Available Neurotoxins. J Clin Aesthet Dermatol. 2014 Feb;7(2):31-39.
Srinoulprasert Y, Wanitphakdeedecha R. Antibody-induced botulinum toxin treatment failure: A review and novel management approach. J Cosmet Dermatol. 2020 Jul 23. doi: 10.1111/jocd.13637. Epub ahead of print. PMID: 32702171.
Zhao K, Guillaud M, Hu A. Factors Associated with Failure of Botulinum Toxin Injection in Adductor Spasmodic Dysphonia. Ann Otol Rhinol Laryngol. 2020 Oct;129(10):996-1002. doi: 10.1177/0003489420928373. Epub 2020 May 29. PMID: 32468829.
Carr WW, Jain N, Sublett JW. Immunogenicity of Botulinum Toxin Formulations: Potential Therapeutic Implications. Adv Ther. 2021 Oct;38(10):5046-5064. doi: 10.1007/s12325-021-01882-9. Epub 2021 Sep 13. PMID: 34515975; PMCID: PMC8478757.
Blumenfeld AM, Stark RJ, Freeman MC, Orejudos A, Manack Adams A. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018 Feb 5;19(1):13. doi: 10.1186/s10194-018-0840-8. PMID: 29404713; PMCID: PMC5799088.
Arbizu RA, Rodriguez L. Use of Clostridium botulinum toxin in gastrointestinal motility disorders in children. World J Gastrointest Endosc 2015; 7(5): 433-437 [PMID: 25992183 DOI: 10.4253/wjge.v7.i5.433]
Bowden JB, Rapini RP. Psoriasiform eruption from intramuscular botulinum A toxin. Cutis 1992;50:415– 416.
Mezaki T, Sakai R. Botulinum toxin and skin rash reaction. Mov Disord. 2005 Jun;20(6):770. doi: 10.1002/mds.20440. PMID: 15747363.
Brüggemann N, Dögnitz L, Harms L, Moser A, Hagenah J. Skin reactions after intramuscular injection of Botulinum toxin A: a rare side effect. BMJ Case Rep. 2009;2009:bcr09.2008.0942. doi: 10.1136/bcr.09.2008.0942. Epub 2009 Feb 2. PMID: 21686551; PMCID: PMC3028521.