Iowa Thoracic Surgery Protocols
Please direct questions and comments to Evgeny V. Arshava, MD evgeny-arshava@uiowa.edu
Also visit :
Empyema and Parapneumonic Effusions: Thoracoscopic (VATS) Drainage and Decortication
Empyema and Trapped lung: Open Drainage and Decortication
Empyema and Parapneumonic Effusions: General Considerations
Introduction
Paraneumonic effusion and empyema are the most common benign conditions involving the pleura. Pneumonias are frequently (20–40%) accompanied by reactive parapneumonic effusions containing thin clear fluid.
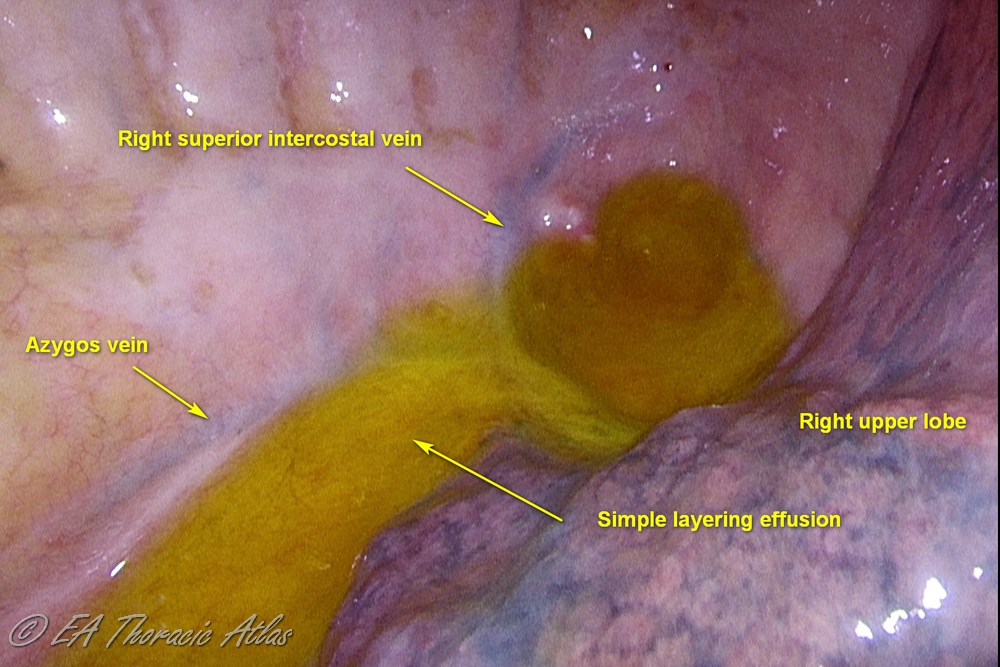
In most cases effusions spontaneously resolve, but in some patients, they may persist and become infected (termed complicated effusions).
Complex effusions are those that have internal loculation.
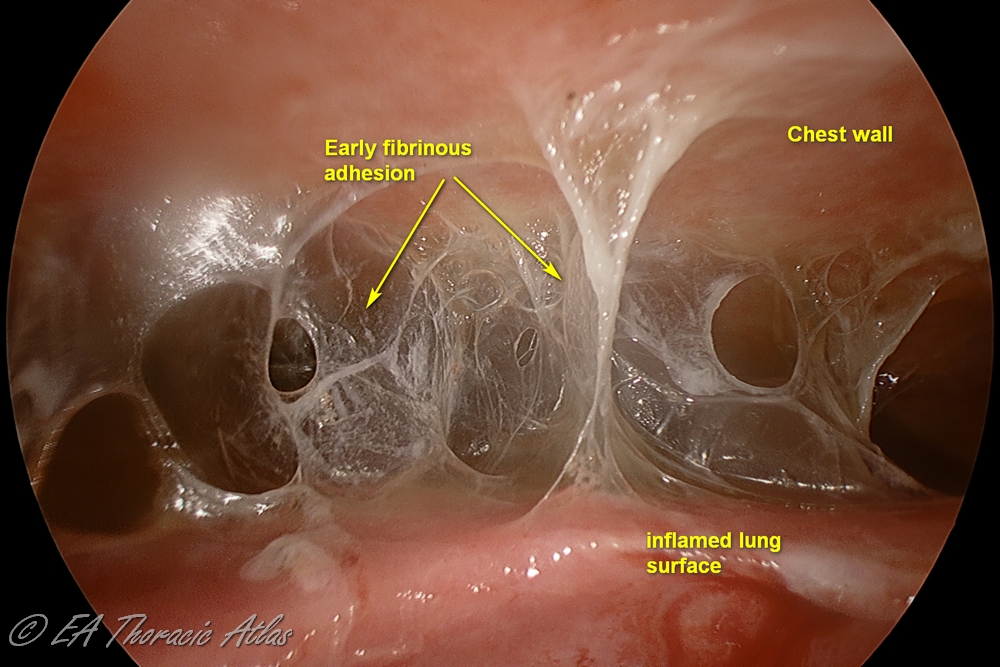
In some cases of complicated effusions, flank pus accumulates in the pleural space and such effusion is called empyema.
Three phases of empyema are classically described:
The exudative phase (Stage I) occurs during first 1-2 weeks of illness when the fluid is free-floating within the thorax and associated with inflamed pleura. Simple drainage with chest tube and intravenous antibiotics are usually sufficient for management.
The fibropurulent phase (Stage II) during weeks 1-4 is characterized by purulent effusion and fibrinous exudative deposits on the pleural surface.
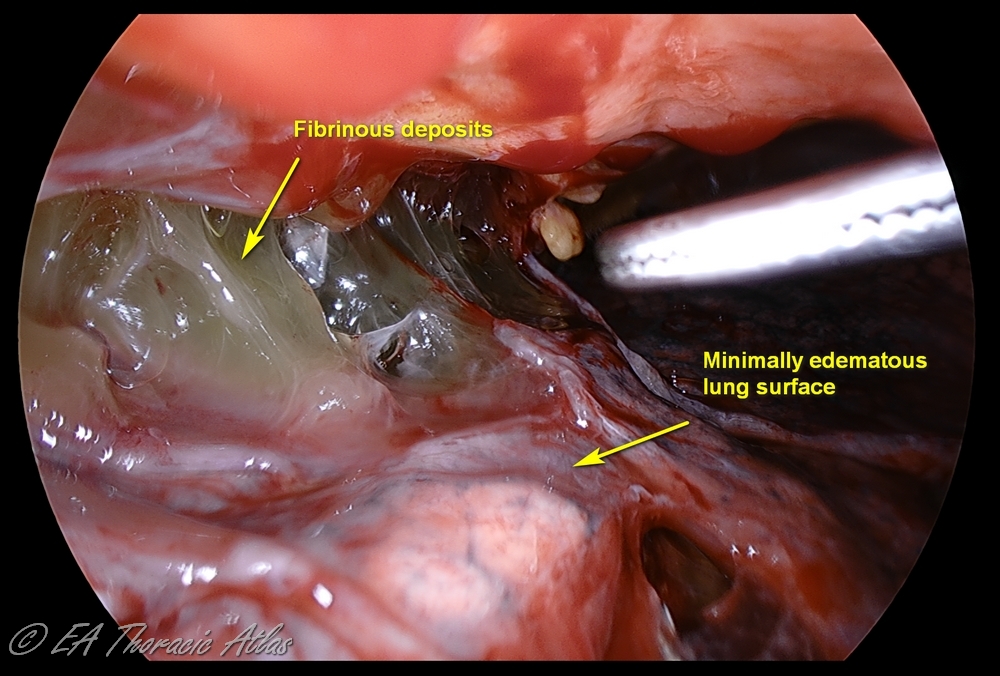
With deposition of protein from the fluid, fibrinous septations form and create loculated areas. Infected fluid becomes highly viscous due to bacterial components and biofilm formation. At this point tube drainage alone is not effective. This stage requires removal of the fibropurulent debris and drain infected fluid.
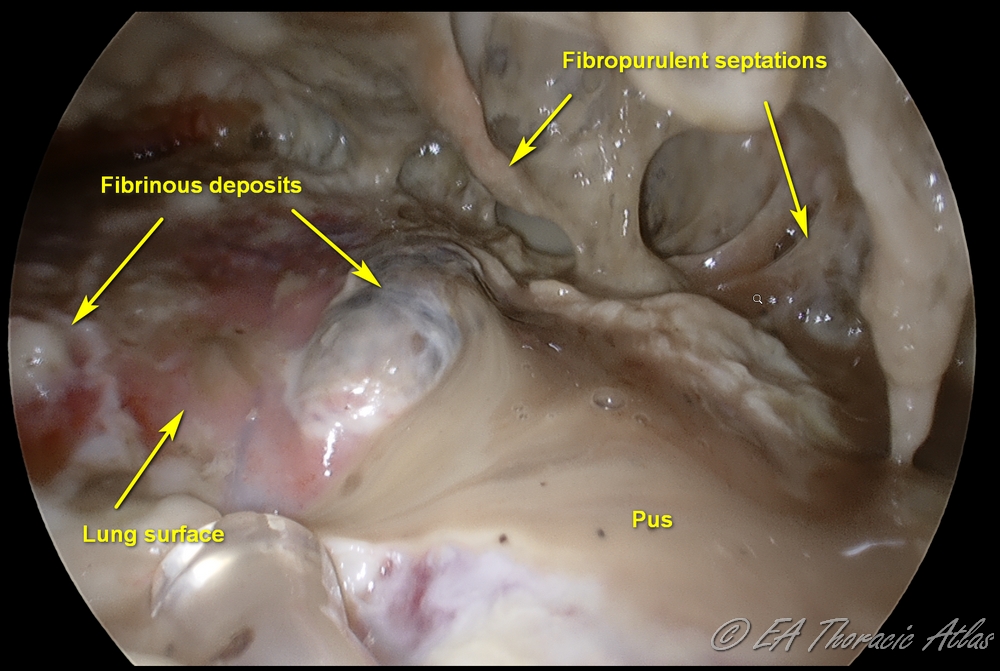
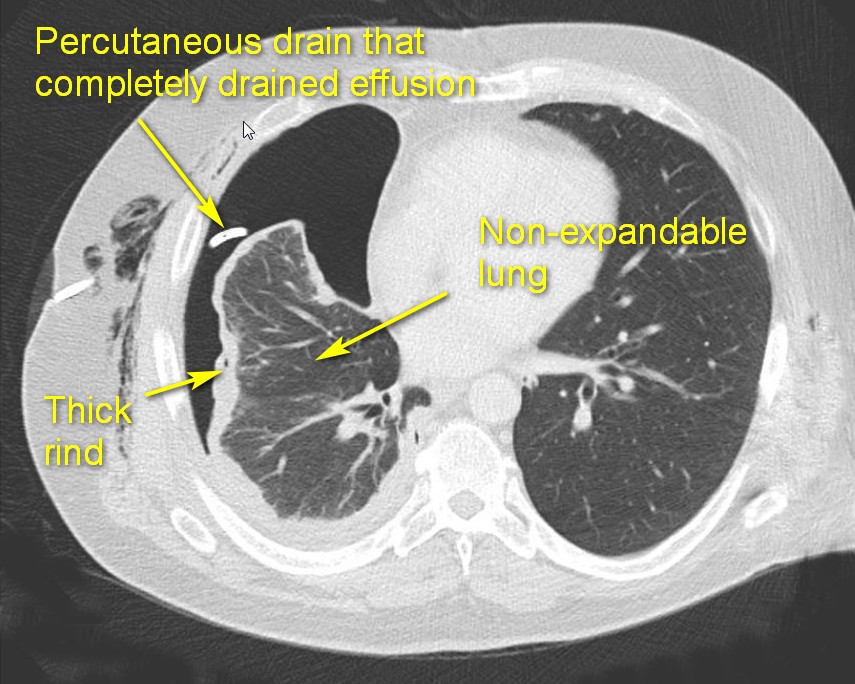
The fibrous phase (Stage III) is best avoided by NOT DELAYING drainage during the earlier stages. This phase starts in 4-6 weeks and is characterized by the formation of a thick, fibrous rind (peel or cortex) on the visceral pleural surface of the lung. This peel traps the compressed lung, serving as a non-expandable “cast.” The lung may become densely adherent to the chest wall and diaphragm (particularly posterior-inferiorly). Parietal pleura becomes fibrotic as well, resulting in intercostal spaces construction and limiting chest wall mobility. Development of this fibrotic process in the chest is called fibrothorax. Even after drainage of the effusion the lung cannot appropriately expand (trapped lung). A thoracotomy and open decortication are usually required to achieve a complete decortication and reexpand the lung.
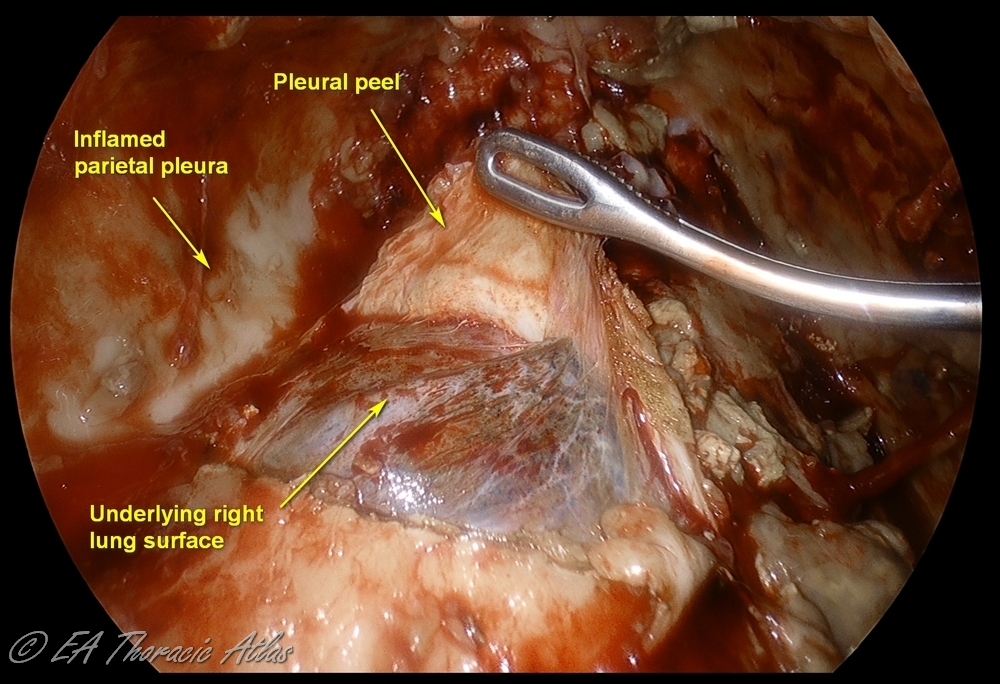
Note that transition between stages in gradual and depends on patient factors, on the causative bacteria and antibacterial treatments. Different areas of the of the chest can be affected differently in any given patient. Most dependent areas (diaphragmatic recess, lower lobe diaphragmatic surfaces) have typically more advanced inflammatory changes.
Complications of empyema tend to occur during the chronic stage and may manifest as bronchopleural fistula, intrathoracic abscess, pericarditis, osteomyelitis.
Cases of empyema necessitans—extension of empyema through the intercostal space into the chest wall outside of the ribs—are fortunately rare at the present day of age.
Thoracentesis and Pleural Fluid Analysis
The differential diagnosis of pleural effusions is broad and includes various benign (infection, trauma, pulmonary embolism, chronic heart failure, connective tissues disorders, chylothorax) and malignant etiologies.
Thoracentesis is commonly used to differentiate transudative versus exudative effusions. In general, an exudative effusion meets at least one Light’s criteria:
●Pleural fluid-to-serum protein ratio greater than 0.5
●Pleural fluid-to-serum lactate dehydrogenase (LDH) ratio greater than 0.6
●Pleural fluid LDH greater than two-thirds of the upper limits of the laboratory's normal serum LDH
Light’s criteria have a high sensitivity but a moderate specificity to identify exudative pleural effusions. For instance, transudates may be incorrectly identified as exudates (diuretics use for heart failure management or malignant effusions with a high level of erythrocytes, releasing LDH).
Thus, while testing of the fluid for Light’s criteria is typically performed, this is rarely helpful in everyday inpatient clinical practice. Most decisions on need for tube or surgical drainage can be made base on imaging alone.
Note that the role of thoracentesis alone should be minimal in the inpatient setting. If pleural fluid sampling is needed, this generally means that the effusion is large enough for safe aspiration. In such cases placement of 14 Fr catheter over the wire does not add any time and does not significantly increase the risks of the procedure. On the other hand, placement of catheter (for instance, in an attempt to use TPA/DNAse) later exposes patients to risks of additional procedure and increases the duration of the hospital stay.
Pleural fluid analysis may help to distinguish empyema from a non-infected effusion based on the positive Gram stain or culture or pH less than 7.2.
Identification of causative bacteria is important to guide antibacterial therapy. Please note that in half of the cases of complicated effusions, pleural fluid and even intraoperative tissues cultures demonstrate no growth (due to patients already receiving antibiotics and limitations of the specimen delivery and handling).
Diagnosis of mycobacterial infection may be especially challenging. Adenosine deaminase (ADA), an enzyme is essential for the function of lymphocytes, and its levels correlate with burdens of mycobacterial antigens in the pleura space. The test is sensitive for diagnosis of tubercular effusion, but not specific enough and may be elevated in other inflammatory conditions.
The best way to confirm diagnosis of tuberculosis is video-assisted thoracoscopic surgery (VATS) pleural biopsy that have close to 80% confirmation of microscopy.
Relevant Imaging
Chest X-Ray (CXR)
- Upright CXR can demonstrate effusion of approximately 200 mL. Thus, smaller fusions (usually irrelevant clinically) may not be seen.
- CXR demonstrates opacity of the hemothorax, but sometimes effusion may be undistinguishable from lung consolidation.
- Free-floating fluid may be identified by repeating the CXR in the decubitus position, by changing its position (layering by gravity). This is less often practiced compared to bedside ultrasound imaging.
- CXR is used to assess the adequacy of fluid drainage and expansion of the lung.
- CXR demonstrates the position of the chest tubes and percutaneous drains (also see chest tubes pitfalls).
- The sentinel hole (the last side hole on the chest tube from the tip) needs to be located within the chest. Note: Centimeter marks on the chest tube define the distance from the sentinel hole and NOT from the tip of the chest tube.
Ultrasonography
- May differentiate atelectasis versus effusion at the bedside.
- Can identify presence of loculations that predicts the failure of tube drainage alone.
- Helps identify the optimal site for thoracostomy tube placement.
Computed Tomography (CT)
- CT is the main diagnostic modality to decide on operative intervention or percutaneous drainage.
- CT readily distinguishes pleural fluid from consolidation. Even in a non-contrast scan the border between the parenchyma and fluid may still be identified by differences in their densities and bronchograms (air in the airways) in the lung parenchyma. Locations and sizes of loculations are defined as well.
- Free floating (layering) pleural effusion and loculations (lenticular shaped opacities) can generally be distinguished on CT. Typically finding of such effusion with concave shape means it is free-floating and can be managed with tube drainage alone.
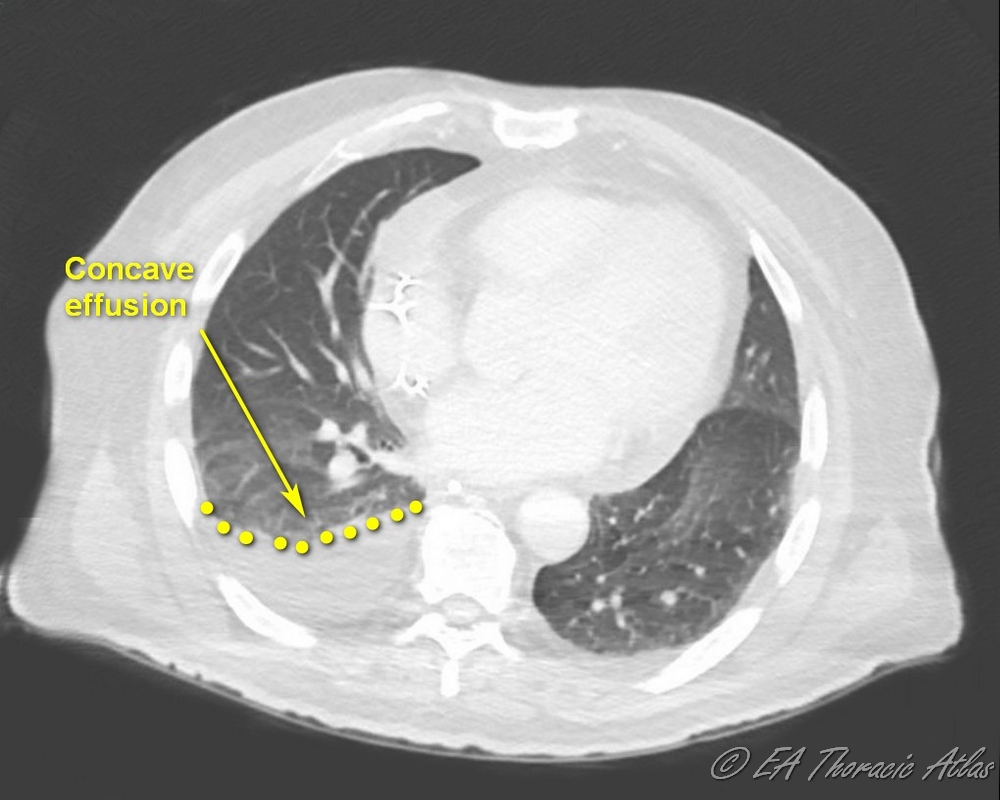
- Effusions with convex shape or multiple loculations generally mean that this is an advanced process with high density fluid that likely requires surgical drainage for efficient management.
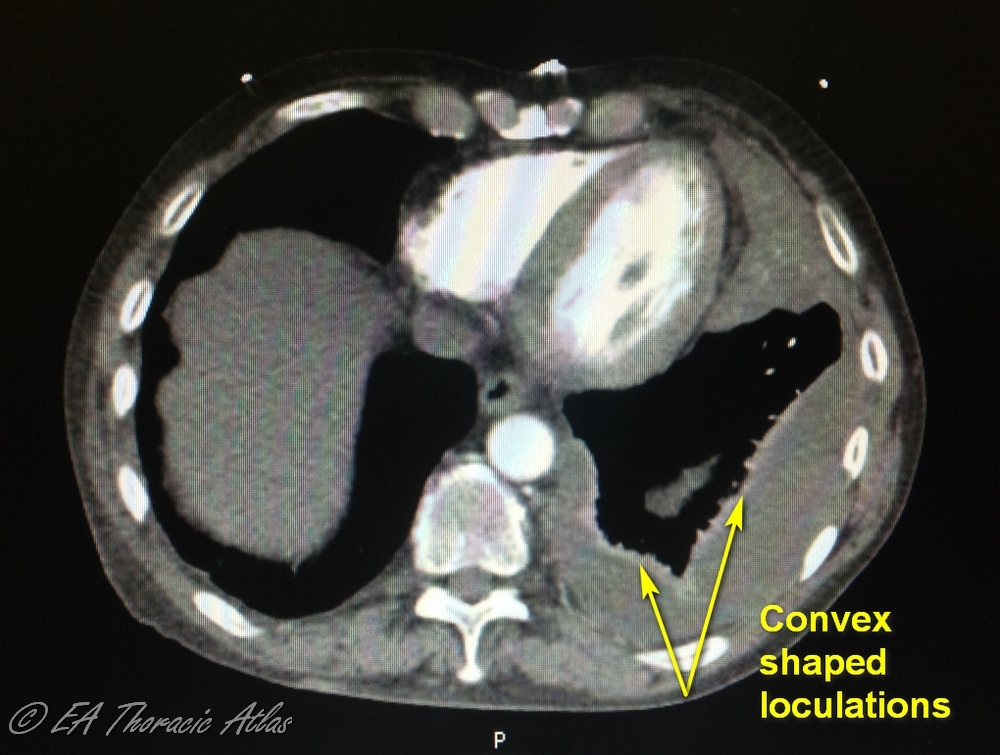
- Presence of discrete pleural rind predicts the likely failure of tube management and need to perform an operation. Lower thickness of pleural rind predicts the success of VATS decortication.
Initial Management
Presentation of patients presenting with pneumonia and effusions may range from minimal constitutional and respiratory symptoms to respiratory failure and sepsis. Management is thus follows the typical ABC (Airway, Breathing, Circulation) algorithm.
Antibacterial Management Principles
- Start empiric antibiotics: community versus hospital-acquired infections
- Consider patient risk factors (immunosuppression)
- If the patient is symptomatic, antibiotics should not be delayed pending diagnostic testing or drainage of the effusion.
- If the patient is minimally symptomatic, consider getting s sample before administration of antibiotics.
- Pursue targeted therapy based on microbiology results.
Community-acquired Cases
Treat the patient with an empiric intravenous (IV) antibiotic with the goal to cover Streptococcus pneumoniae, oropharynx flora streptococci (e.g., Streptococcus anginosus, Streptococcus intermedius) and anaerobic bacteria
Consider a third-generation cephalosporin (e.g., ceftriaxone or cefotaxime) plus metronidazole
or
beta-lactam/beta-lactamase inhibitor combination (e.g., ampicillin-sulbactam)
For patients allergic to penicillin, instigate monotherapy with a carbapenem (imipenem, meropenem), monobactam (e.g., aztreonam), plus metronidazole
Hospital-acquired Cases
Treat the patient with an empiric IV antibiotic regimen that targets methicillin-resistant Staphylococcus aureus (MRSA), Gram-negative bacteria (including Pseudomonas spp), and anaerobic bacteria
Consider vancomycin with metronidazole and an antipseudomonal cephalosporin (cefepime, ceftazidime)
or
vancomycin with an anti-beta-lactam/beta-lactamase inhibitor (piperacillin-tazobactam)
For patients allergic to penicillin, prescribe a antipseudomonal fluoroquinolone (ciprofloxacin)
Duration of Therapy
- The optimal duration of therapy is not known.
- Self-resolving uncomplicated bacterial parapneumonic effusions à 1–2 weeks.
- Complicated parapneumonic effusion à 2–3 weeks.
- Empyema à 4–6 weeks.
- IV antibiotic regimen can be switched to an oral regimen upon clinical improvement.
Respiratory Care
should be comprehensive and aggressive and include:
- ABC
- Sepsis management
- Pulmonary care: incentive spirometry, flutter valve, chest physical therapy
- Bronchodilators
- Mucolytics
- Ambulation and physical therapy
- Pain management (in the setting of rib fractures or presence of surgical incisions/drains)
Indications for Drainage
Most uncomplicated, parapneumonic, small (<2–2.5 cm) pleural effusions that are free-floating resolve with the appropriate treatment of the pneumonia.
Accepted indications for an effusion drainage based on thoracentesis results are the following:
- Purulence or positive Gram stain
- Pleural pH <7.2
- Pleural glucose <40 mg/dL
- Pleural LDH >1000 IU/L
Consider drainage in cases of
- Symptomatic affusions
- Adjacent atelectasis seen on CT
- Limited baseline respiratory reserve
- Worsening clinical or radiographic features
- Large (approaching to one-half of the hemithorax) or multiloculated pleural effusions, regardless of presence of infection, need to be drained (with tubes or surgically) to allow maximal re-expansion of the lung.
The persistence of loculated effusions may depend on their etiology (which bacterial pathogen), appropriateness of antibacterial treatment, and an individual patient's physiologic and anatomic characteristics. Failure to drain the effusion results in progression of the inflammatory process, entrapped lung, and long-term decreased respiratory capacity.
Tube Thoracostomy
- Image guidance is beneficial especially with loculated effusions: ultrasound or CT.
- Tube thoracostomy works for non-septate collections.
- There is no consensus on the size of chest tube or catheter for drainage, as guidelines vary on their recommendations.
10–14 Fr cause less pain than larger-diameter tubes.
We suggest 14 Fr as the minimum size for drainage (they clog and kink less frequently).
“Pigtails” – dislodge out of the appropriate position less frequently than straight tubes of the same diameter.
- Timing of tube removal:
Output <100 ml/day for clear effusions.
<50 ml/day for empyema
Management controversy
There is an ongoing debate on the best approach to the management of complicated parapneumonic effusions and empyema (see Suggested Reading below).
Larger retrospective studies have many limitations, including the surgical candidacy selection process and lack standardization of various parameters. Randomized studies on direct comparison surgery versus intrapleural enzyme therapy are only beginning to appear.
MIST I (Multicenter Intrapleural Sepsis Trial)
streptokinase did not decrease mortality, need for surgery, or length of stay. The need for operation was 30%.
MIST II
Intrapleural tPA/DNase combination decreased hospital stay (6.7 days)
Each agent alone was not effective compared to placebo.
True for purulent versus non-purulent effusions, large- versus small-bore drainage tubes, loculations versus no loculations.
80% decrease (4% versus 16 %) in need for surgery
MIST III (small number of patients studied)
Earlier resolution of pain with intrapleural tPA/DNase combination
Potential shortening of length of stay with early operation.
The American Association for Thoracic Surgery consensus guidelines for the management of empyema 2017
Intrapleural fibrinolytics should not be used routinely for complicated pleural effusions and early empyema.
VATS should be the first-line approach in all patients with stage II acute empyema.
Open decortication is reasonable in patients with chronic empyemas who are medically operable to tolerate major thoracic surgery.
Current Best Practice on Thoracostomy Tube Management versus Surgery
- Most cases of free-floating paraneumonic effusion and some cases of early empyema may be resolved with tube thoracostomies (surgical chest tube or percutaneous drain).
- In some cases, intrapleural instillation of tissue plasminogen activator (t-PA) and human recombinant DNase through the thoracostomy tube may facilitate the resolution of empyema if loculations are present. This non-operative approach may be associated with a longer hospital stay and need for re-interventions.
- Intrapleural tPA/DNase may decrease need for surgery in some patients, but certainly not all. Early thoracoscopic surgery is a more efficient approach in an average risk patient and is associated with shorter length of hospital stay.
- Thoracic surgeons need to be involved early in the evaluation of patients. Routing initial use of intrapleural tPA/DNase may be considered on case-by-case bases, only multidisciplinary discussion.
- VATS offers advantage over the chest tube thoracostomy and intrapleural t-PA/DNase in cases of multiloculated effusions.
- Loculated purulent fluid can be completely drained under direct vision during a single, short operation.
- The entire lung surface is mobilized for optimal lung expansion. Decortication is performed as needed.
- Chest tubes can be placed in the appropriate position for optimal drainage in the postoperative period.
- The need for reinterventions after adequate VATS as compared to percutaneous tube management strategy is uncommon.
- VATS is associated with a short postoperative hospital stay.
- Intrapleural enzymatic management requires interval CT imaging (at times more than one). After adequately performed VATS drainage, most commonly only immediate and outpatient follow-up CXRs are needed.
- We limit percutaneous drainage and intrapleural t-PA/DNase as a definitive approach to high-risk patients for general anesthesia and single-lung ventilation. We also consider an intrapleural fibrinolytics approach for selected cases of early-stage small to moderate volume loculated effusions, where the amount of planned lung expansion does not outweigh the risks of an operation.
Suggested Reading
Shen KR, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg. 2017 Jun;153(6):e129-e146. doi: 10.1016/j.jtcvs.2017.01.030. Epub 2017 Feb 4. PMID: 28274565.
MIST I
Maskell NA et al; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005 Mar 3;352(9):865-74. doi: 10.1056/NEJMoa042473. Erratum in: N Engl J Med. 2005 May 19;352(20):2146. PMID: 15745977.
MIST II
Rahman NM, et al; Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011 Aug 11;365(6):518-26. doi: 10.1056/NEJMoa1012740. PMID: 21830966.
MIST III
Bedawi EO, et al; Early Video-assisted Thoracoscopic Surgery or Intrapleural Enzyme Therapy in Pleural Infection: A Feasibility Randomized Controlled Trial. The Third Multicenter Intrapleural Sepsis Trial-MIST-3. Am J Respir Crit Care Med. 2023 Dec 15;208(12):1305-1315. doi: 10.1164/rccm.202305-0854OC. PMID: 37820359; PMCID: PMC10765402.
Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev. 2017 Mar 17;3(3):CD010651. doi: 10.1002/14651858.CD010651.pub2. PMID: 28304084; PMCID: PMC6464687.
Farjah F, Symons RG, Krishnadasan B, Wood DE, Flum DR. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg. 2007 Feb;133(2):346-51. doi: 10.1016/j.jtcvs.2006.09.038. Epub 2006 Dec 29. PMID: 17258562.
Semenkovich TR, Olsen MA, Puri V, Meyers BF, Kozower BD. Current State of Empyema Management. Ann Thorac Surg. 2018 Jun;105(6):1589-1596. doi: 10.1016/j.athoracsur.2018.02.027. Epub 2018 Mar 14. PMID: 29550205; PMCID: PMC5964038.
Chaddha U et al. Use of fibrinolytics and deoxyribonuclease in adult patients with pleural empyema: a consensus statement. Lancet Respir Med. 2021 Sep;9(9):1050-1064. doi: 10.1016/S2213-2600(20)30533-6. Epub 2021 Feb 2. PMID: 33545086.
Akulian J, et al; Interventional Pulmonary Outcomes Group. Bleeding Risk With Combination Intrapleural Fibrinolytic and Enzyme Therapy in Pleural Infection: An International, Multicenter, Retrospective Cohort Study. Chest. 2022 Dec;162(6):1384-1392. doi: 10.1016/j.chest.2022.06.008. Epub 2022 Jun 16. PMID: 35716828; PMCID: PMC9773231.