Iowa Thoracic Surgery Protocols
Please direct questions and comments to Evgeny V. Arshava, MD evgeny-arshava@uiowa.edu
Also see
Empyema and Parapneumonic Effusions: General Considerations.
Empyema and Trapped lung: Open Drainage and Decortication
Open window thoracostomy
Indications for surgical empyema and parapneumonic effusions drainage / decortication
- Multiloculated pleural effusions
- Fibropurulent phase of empyema (most cases are successfully managed with VATS)
- Fibrous phase of empyema (usually requires thoracotomy)
- Patients with empyema presenting with sepsis, septic shock or acute respiratory failure need to be managed initially with tube thoracostomy, antibiotics and supportive care. Operative drainage and decortication should be postponed until clinical condition stabilizes.
Please note that in case of delayed presentation of chronic effusion or chronic empyema with a trapped lung 4-6 weeks after onset of symptoms, immediate decortication on the peak of inflammatory changes may be complicated with increased risk of air leak and bleeding. In these cases, it may be better to delay elective decortication for few weeks to allow the peak of inflammatory changes in the chest to subside.
Note that most tuberculous effusions will resolve with appropriate antibacterial treatment and pleural drainage should be used selectively.
Thoracoscopic (VATS) versus Open (Thoracotomy) Empyema Drainage and Lung Decortications
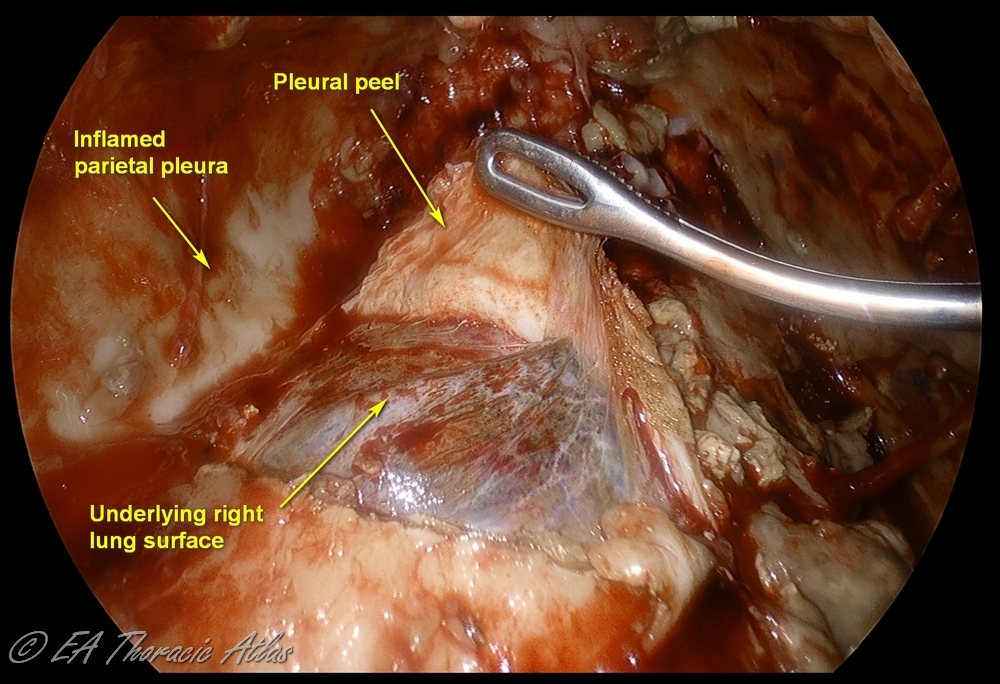
- Suctioning and evacuation of the fluid and fibrinopurulent debris from the pleural space id called “Drainage”. Removal of the discrete fibropurulent peel present on the lung and chest wall is called “Decortication”. While this terms commonly used interchangeably, they involved separate technical manipulations.
- Thoracoscopy (VATS) should be the first line approach in all patients with early-stage empyema.
- VATS approach decreases postoperative pain, decreases blood loss, shortens hospital stay decreases respiratory and other postoperative complications and 30-day mortality.
- There is no downside to attempt VATS drainage/decortication as an initial approach and convert intraoperatively top thoracotomy is anatomy and inflammatory changes are not favorable for minimally invasive approach.
- Robotic assisted decortication has been describe, but are not practical in everyday practice in our opinion.
- Late stages of empyema and fibrothorax require thoracotomy to release trapped lung.
- Need for open window thoracostomy is very rare at present time, but is an effective bail out approach for cases of chronic empyema, especially postoperative.
Contraindications
- Empyema stage III with fibrothorax when VATS is not efficient and safe.
- Prior chest radiation and operations
- Endobronchial obstruction (tumor) with inability to reexpand the lung
- Chronic respiratory failure with ventilation dependence
- Inability to carry single lung ventilation in a patient with limited cardiopulmonary reserve.
- Malignant pleural disease
Potential Complications
- Lung injury with prolonged air leak
- Bleeding and postoperative hemothorax (from to lung injury or due to perioperative hypothermia and coagulopathy)
- Failure of lung re-expansion and recurrent empyema (usually due to failure to completely mobilize and decorticate the lung, including fissure, and drain all loculations
- Phrenic nerve injury
- Diaphragm injury
- Esophageal injury
Preoperative Preparation
- Patients cardiopulmonary condition optimized. Septic patients can be initially managed with tube thoracostomy until stable. Euvolemia and normothermia must be achieved. Sepsis needs to be resolved prior to thoracoscopy.
- Preoperative CT scan of the chest allows identification of loculated fluid pockets and identification of co-existing pathology.
- In cases where CT clearly shows complicated effusions (multiloculated), preoperative thoracentesis is not indicated.
- Empiric intravenous (IV) antibiotics are started preoperatively to treat underlying pneumonia. They may be adjusted based on microbiology reports if thoracentesis was done previously.
- We do not recommend placing preoperative chest tubes, unless the patient is clinically septic or in respiratory distress due to a very large effusion. Presence of fluid makes the chest entry safer with less of a risk for lung injury.
- As no lung resection is planned, pulmonary function tests are not needed.
Anesthesia Considerations
- Double-lumen endotracheal tube
- Paravertebral block versus intercostal block versus epidural (in cases of high likelihood of conversion to thoracotomy)
- Two large-bore peripheral IV accesses
- Radial arterial line in high-risk patients
- Type and cross
- Patients are usually already on an established antibiotic regimen. If not, then administer IV antibiotic within 1 h of the incision.
- Maintenance of normothermia with termperature >36.5 °C. Hypothermic patients promptly develop coagulopathy and significant bleeding from large, raw surfaces of the chest wall and lung.
Specific Considerations
- The patient is placed in the decubitus position on the bean bag.
- Thorough airways suction (bronchoscope assistance is very helpful) is performed after intubation and before extubation. Empyema patients frequently have copious secretions that may prevent the lung from adequate reexpansion postoperatively.
Instruments and Equipment Considerations
- Thoracoscopic clamps and tray
- Large-bore (10 mm) curved suction
- Thoracoscopic irrigation cannula
- Standard prep in decubitus position using chlorhexidine solution or detidine. Field includes supraclavicular area to below costal margin, spine to nipple line.
- Transparent incise drape (optional)
OPERATIVE PROCEDURE
Goals of the operation:
- Drain all fluid collections.
- Mobilize entire surface of the lung parenchyma including interlobar fissures
- Decorticate (remove) fibropurulent exudate from the lung and diagrammatic surfaces to maximally expand the lung
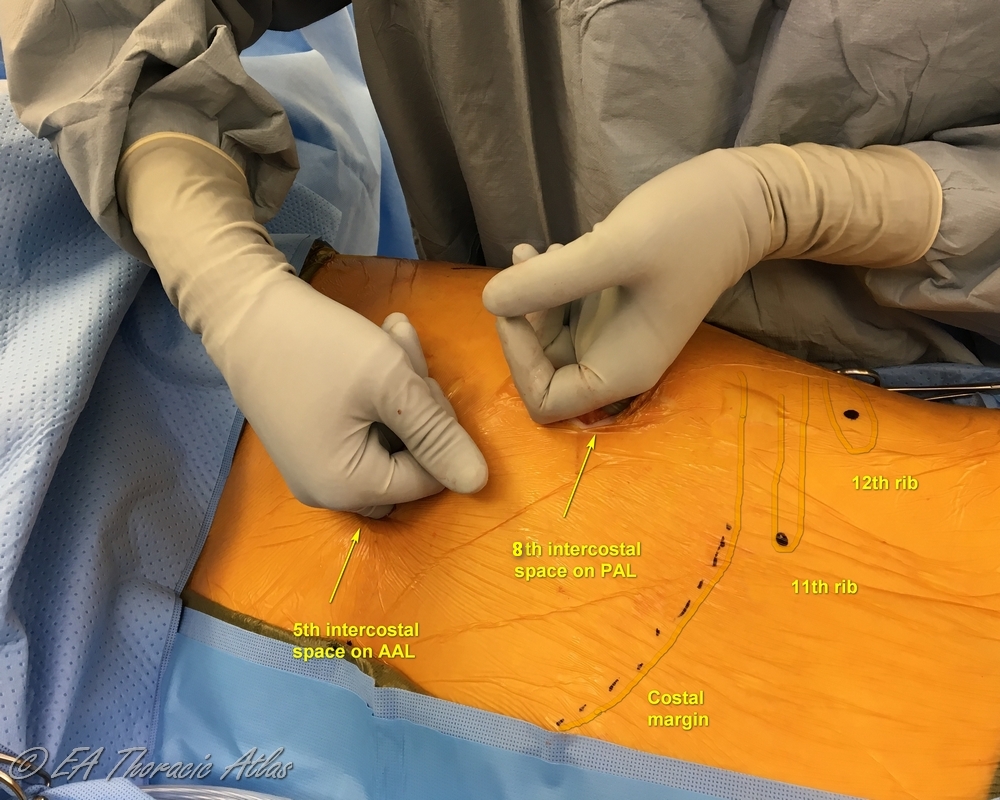
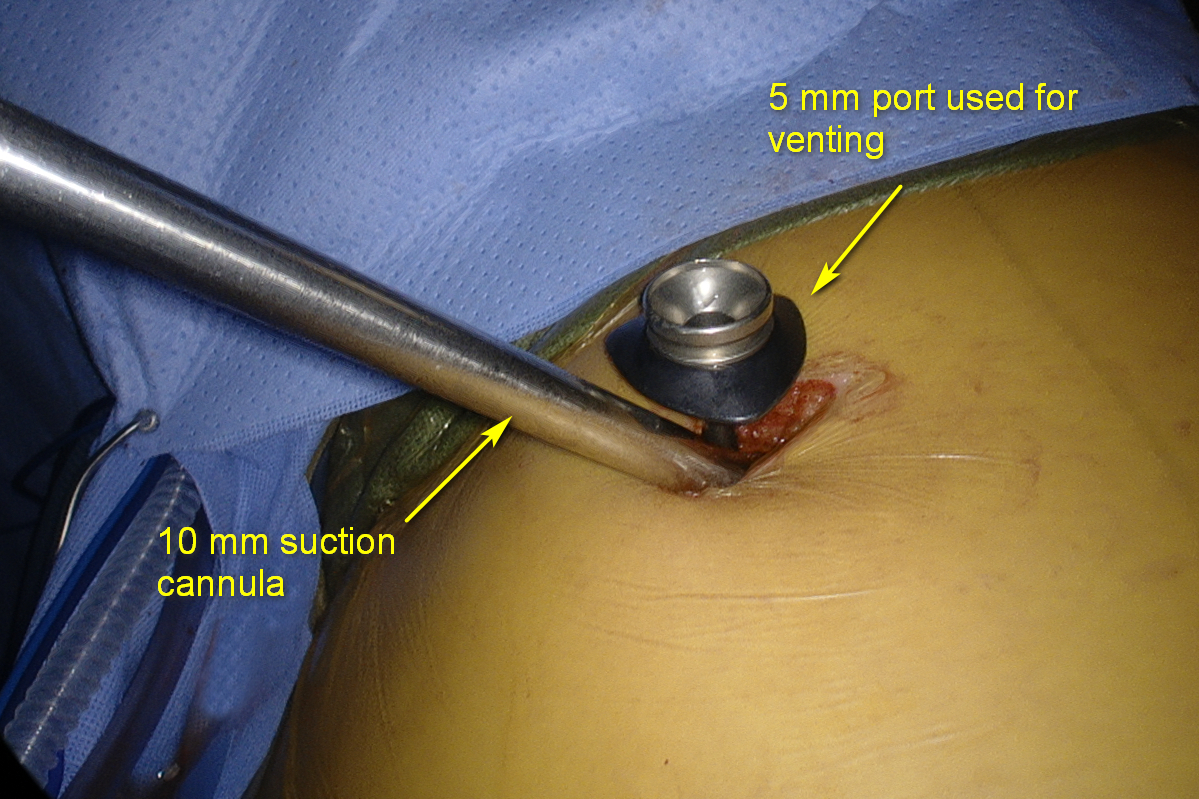
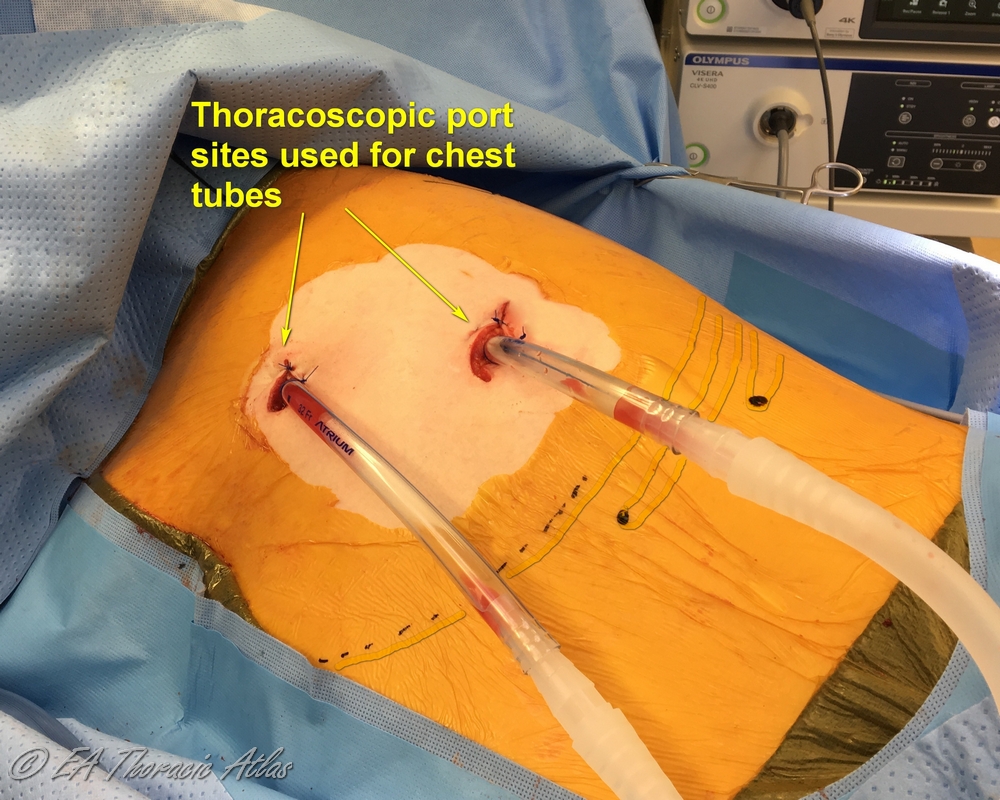
Most thoracoscopic decortications operations can be performed through two 10–15 mm incisions (that postoperatively later serve as tube thoracostomy sites):
- Camera port—7th or 8th intercostal space on the posterior axillary line. Suction or retraction tools may be used through this site along the camera with the port pulled up.
- Working port—4th or 5th intercostal space on the middle or anterior axillary line.
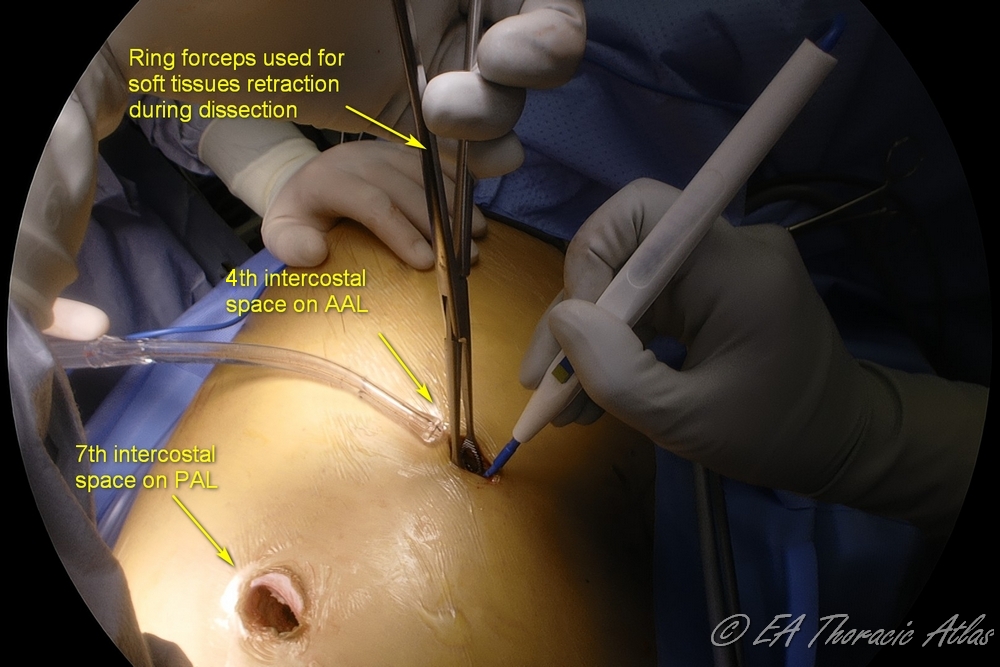
- If the chest tube is already present, its site can be used as one of the port sites.
- Very rarely an additional third port is needed.
- It may be best to avoid posterior ports because intercostal rib spaces posteriorly are narrower and may have higher likelihood of postoperative neuralgia.
- Preoperative imaging may dictate where the first port is best placed (in the area of free fluid to avoid trauma to the lung)
All instruments used in the chest should ideally be curved to accommodate the shape of the chest cavity.
Drainage
- Once the chest is entered at the initial incision, finger dissection with the middle (longest) finger is performed to break lung and diaphragmadhesions to the chest wall as wide as possible in all directions to create working space
- Generally the 2nd incision and port are placed at the maximal reach of the finger dissection.
- Once the camera introduced, the fluid and loose debris are suctioned out to create a working space.
- Lung is further mobilized to increase working space: use gentle blunt dissection close to the chest wall/lung adhesion plane with a ringed clamp or suction.
- Constantly repositioning the dissecting tool closer to the site of adhesions decreases trauma to the lung.
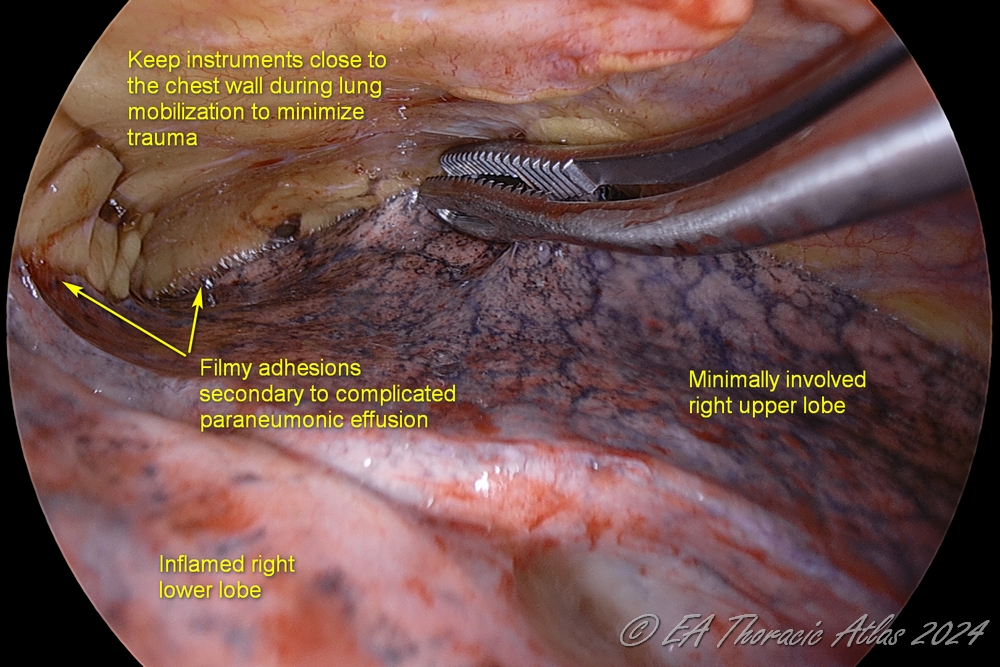
- Use long curved suction tips because they allow for better reach in the convex chest cavity. No less than 10 mm suction should be used for effective evacuation.
- Suction is initially set at 100 -150 mmHg and increased as needed. Higher suction may pull up the lung and decrease the working space. A venting cannula placed along the camera, or instruments may avoid this suction effect.
- Larger fragments of fibrin are separated from the lung and chest wall and removed using ringed clamps.
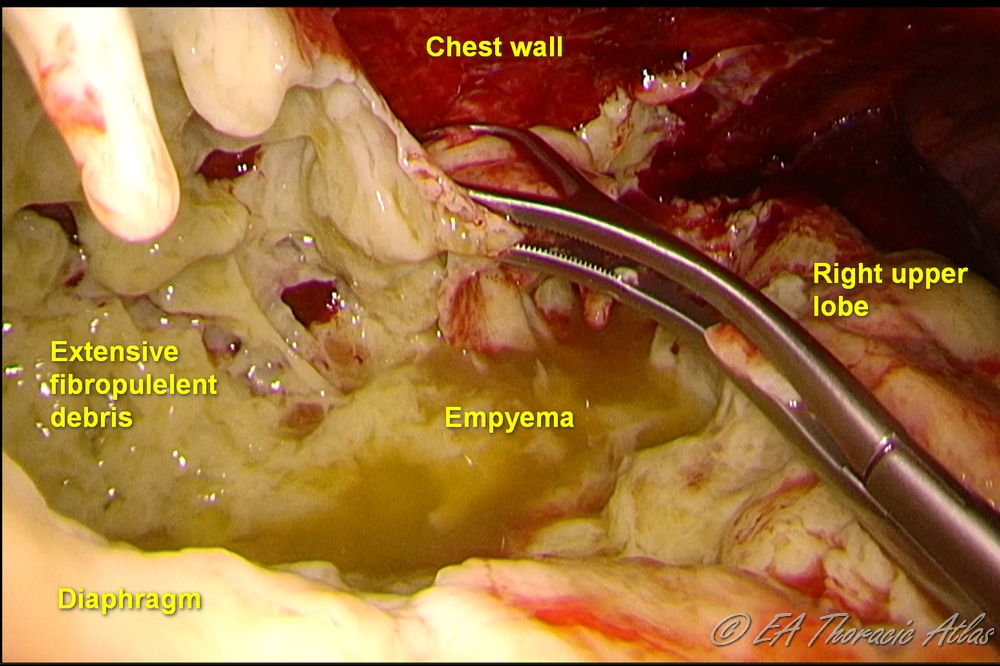
- Samples or fibrinous rind and of all suspicious tissues should be sent for pathological (frozen or permanent depending on the circumstances) and microbiologic examination.
- Throughout the operation washout of the chest with large volumes of warm sterile water through the thoracoscopic irrigation cannula is performed to facilitate evacuate fibropurulent debris and blood clots. Antibiotics irrigations are not necessary.
Mobilization
- Once most fluid and debris has been removed to create adequate working space, full lung mobilization is performed.
- Dissection is performed with clamps and suction tips. Occasionally in patients with thin chest wall and lack of barrel shaped chest, difficult and sensitive areas (fissure, anterior hilum and lower lobe/diaphragm interface can be freed up with finger dissection under direct vision). Educated finger is the safest instrument sometimes.
-
All loculations need to be drained during mobilazation of the lung. Small isolated pus pockets can be occasionally encountered. Computed thomography may at times facilitate their identification preoperatively.
-
For practice purposes, there are 5 components of lung mobilization (exact sequence varies based on the particular case):
-
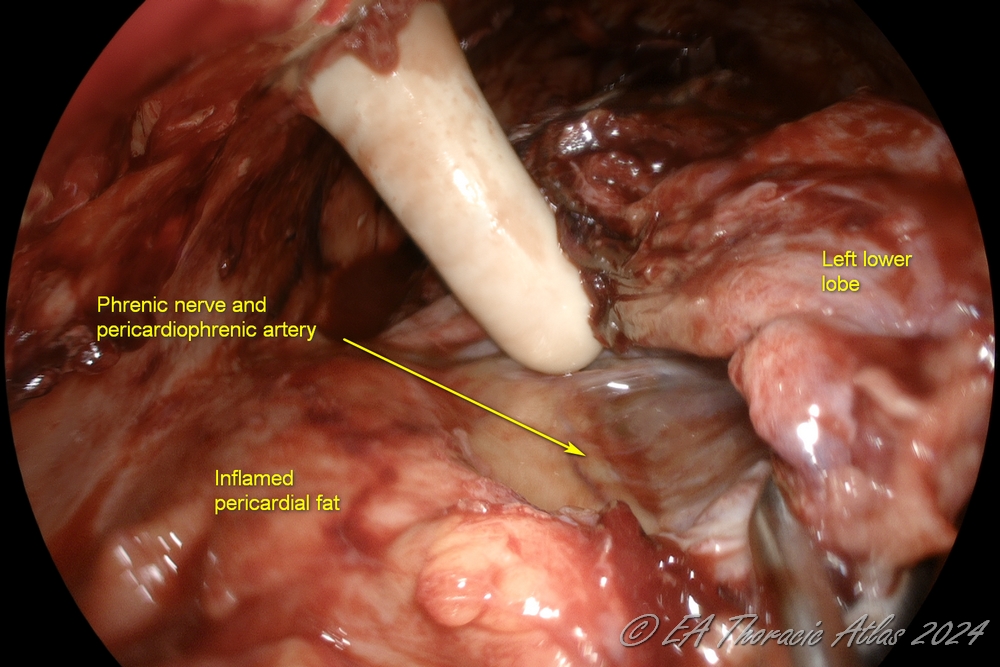
Mobilization anteriorly to dissect the lung away from the heart (protecting the phrenic nerve and internal mammary vessels), exposing the anterior hilum.
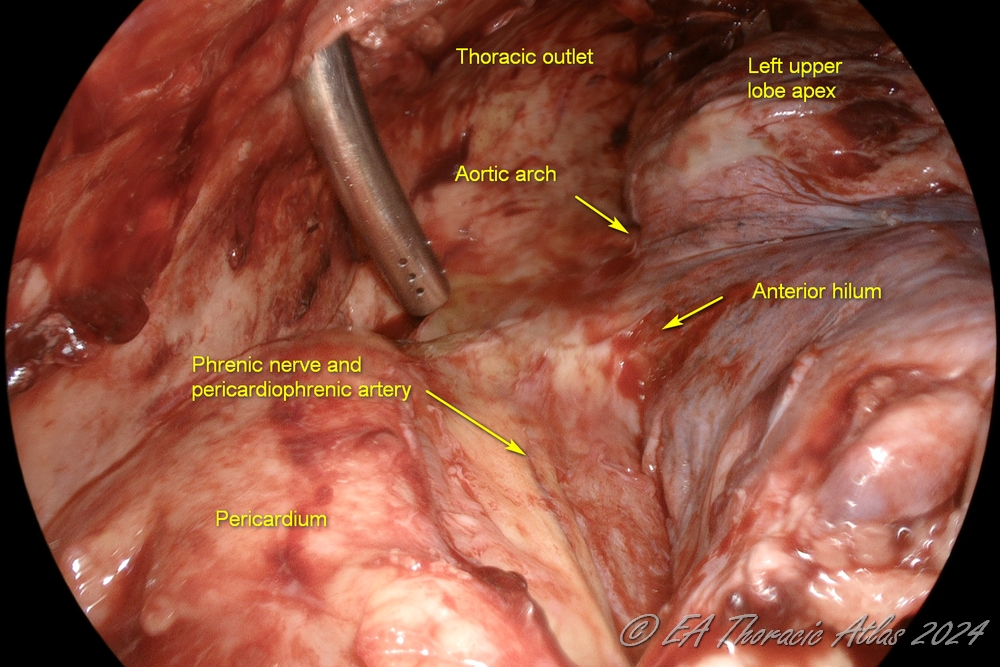
2. Dissection posteriorly to dissect the lung away from the spine and exposing the posterior hilum (protecting the aorta in the left and the esophagus in the right chest
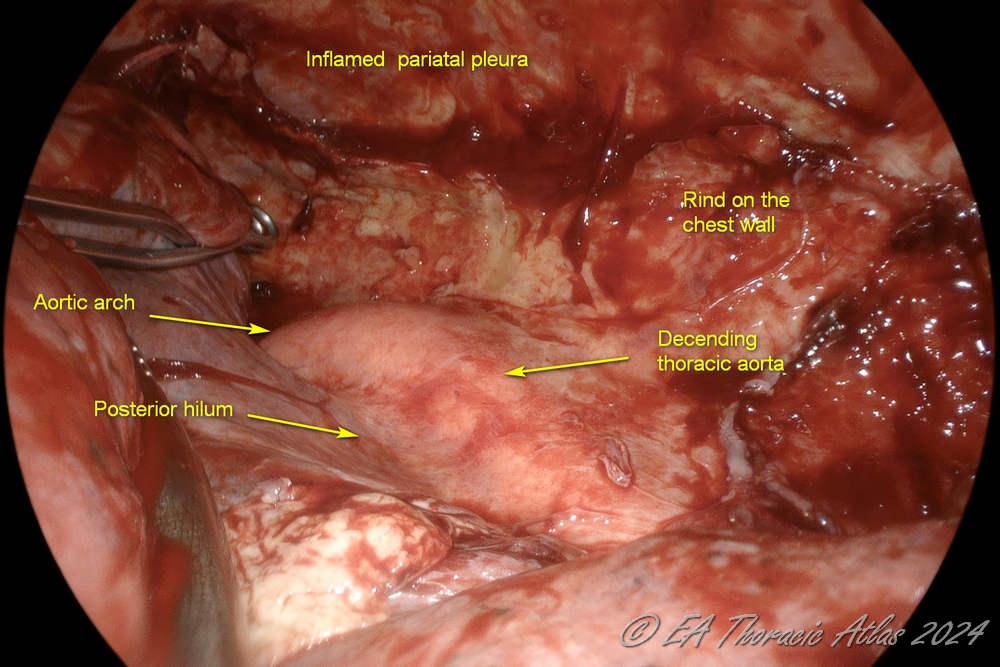
3. Anterior and posterior planes are connected superiorly to free up the apex of the upper lobe (protecting subclavian vessels and the azygos vein).
4. The lower lobe is dissected off the diaphragm (protecting phrenic nerve and diaphragm). The lower lobe /diaphragm plane is most commonly the most inflamed area, and care must be taken to avoid deep lung lacerations. The diaphragm may be pushed down with a ring forceps as needed. Division of the inferior pulmonary ligament is not needed.
- The diaphragm is dissected of the chest wall to its attachments to lower ribs to optimize its respiratory amplitude.
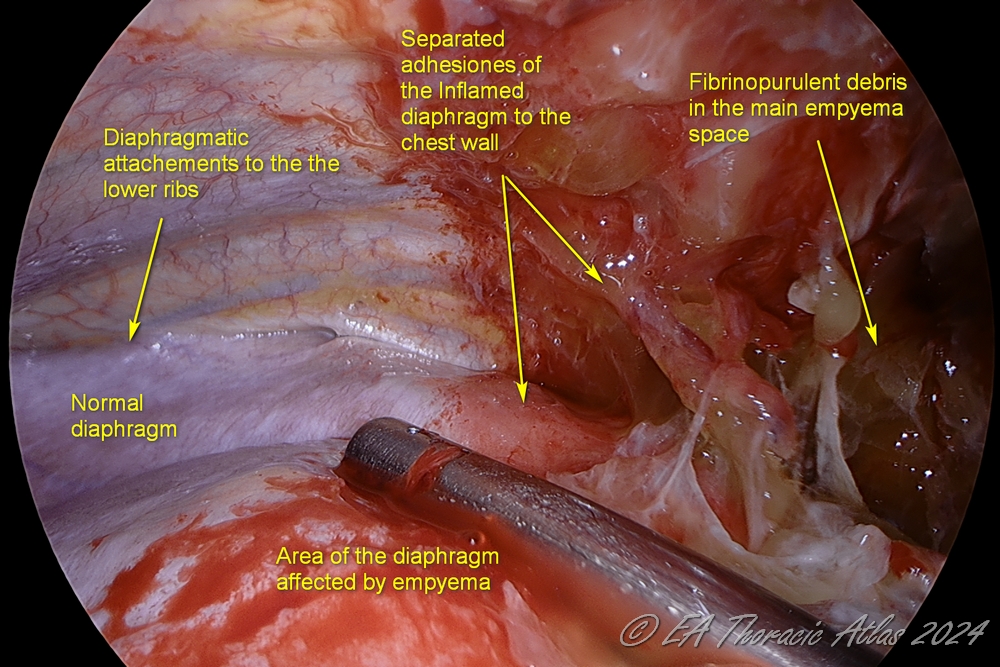
5. Interlobar fissures are freed up.
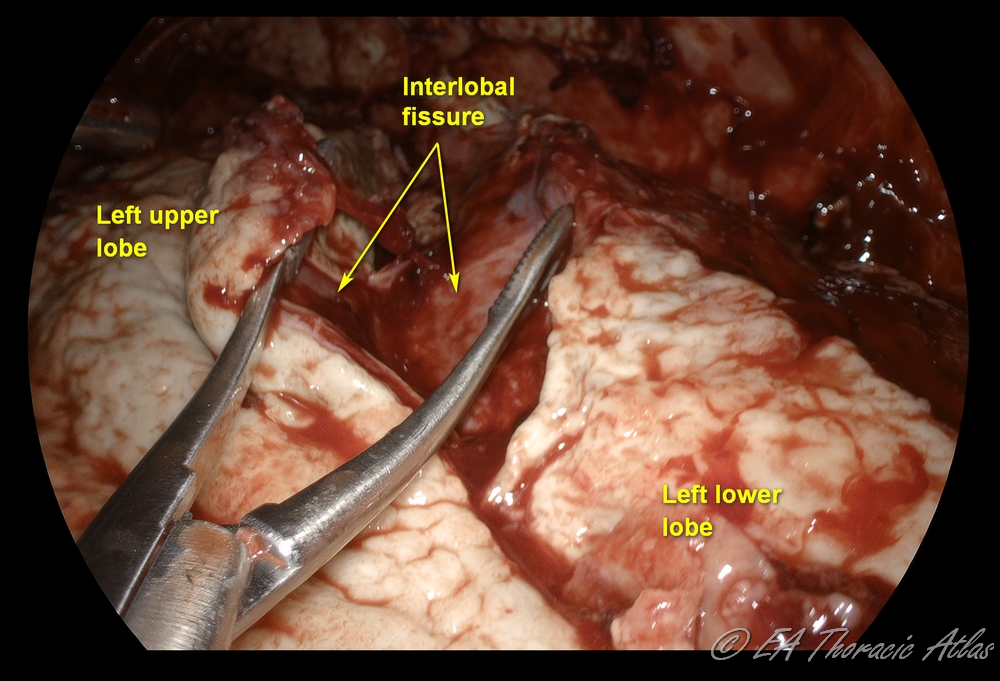
- During mobilization the operating table can be rotated right and left to facilitate exposure as needed.
-
All loculations need to be drained during mobilization of the lung. Small isolated pus pockets can be occasionally encountered. Computed tomography may at times facilitate their identification preoperatively.
Decortication
- Maintain the patient’s temperature at or above 36.5 °C. Hypothermia during decortication due to large raw surfaces promptly leads to coagulopathy and significant blood loss.
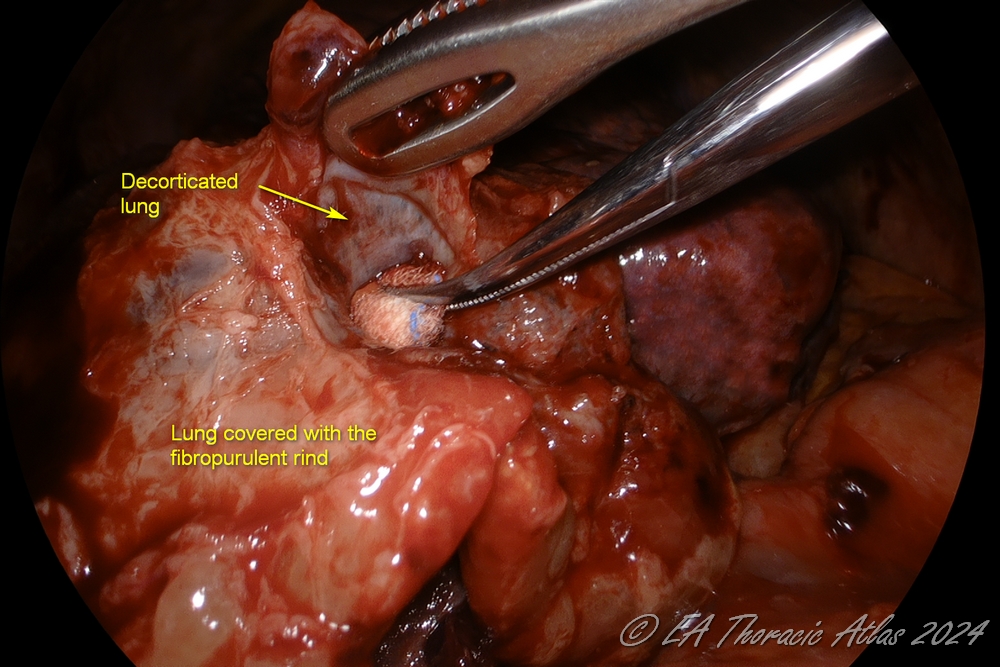
- Decortication is performed to remove adherent fibropurulent exudate or fibrotic rind covering the mobilized lung surfaces. Maximally possible decortication should be performed to expand the lung.
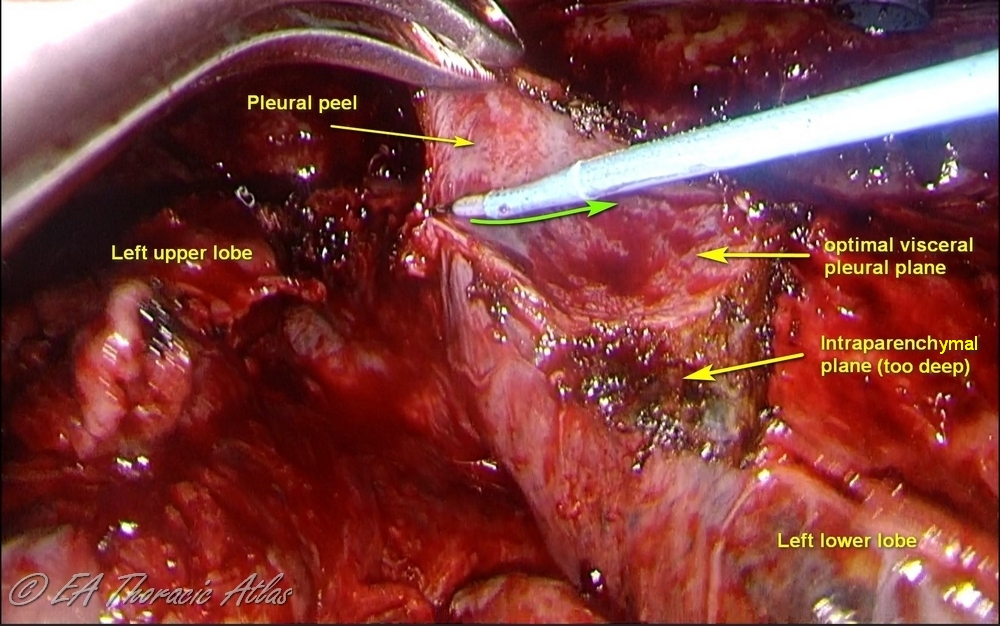
- Various technical maneuvers can be used that work best in different cases depending on the prominence of the rind and its adherence to the underlying lung.
- Thin peel can be removed from the lung surfaces using suction tips.
- Use serrated forceps to removed thicker peel off the lung (retract lung with atraumatic ring forceps).
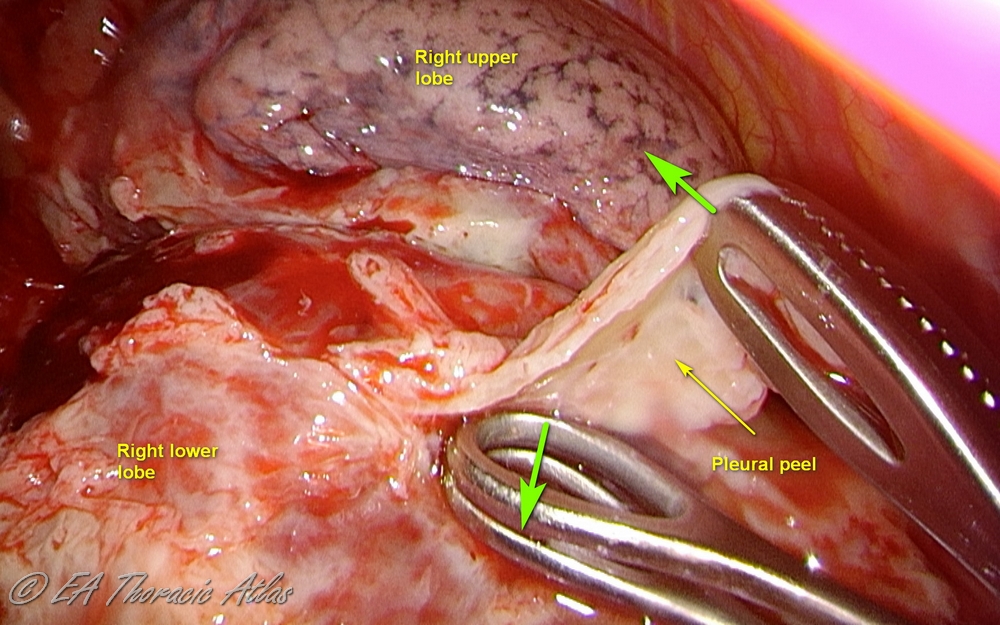
- Counter tension with another instrument makes process more efficient (suction, another clamp or the small “peanut” sponge can be used)
- Dissection of fibrotic rind may require electrocautery. Care must be taken to find optimal depth of dissection to decrease trauma to underlying lung.
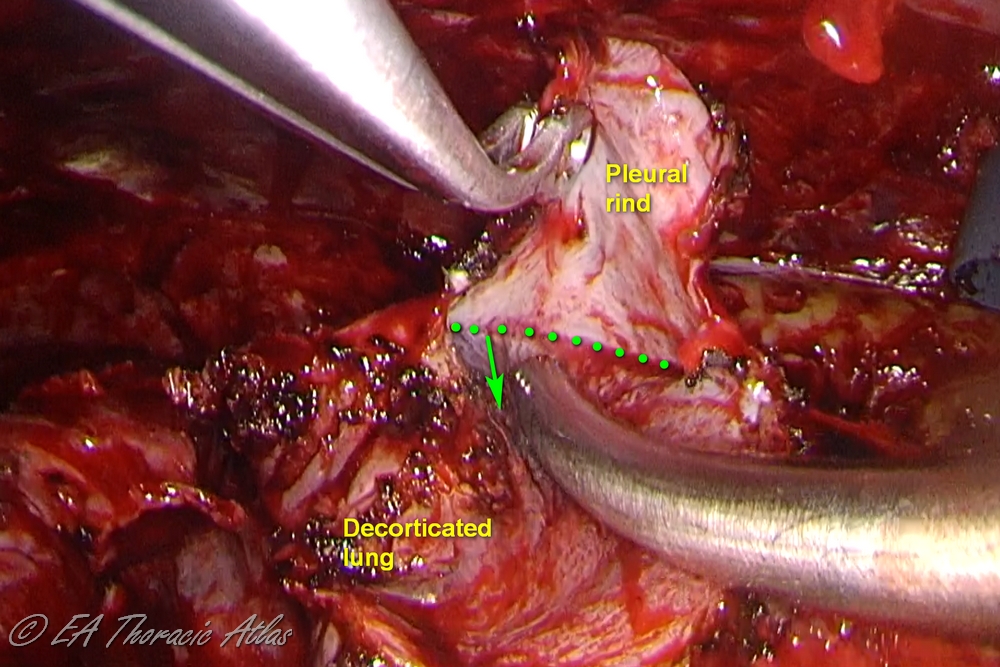
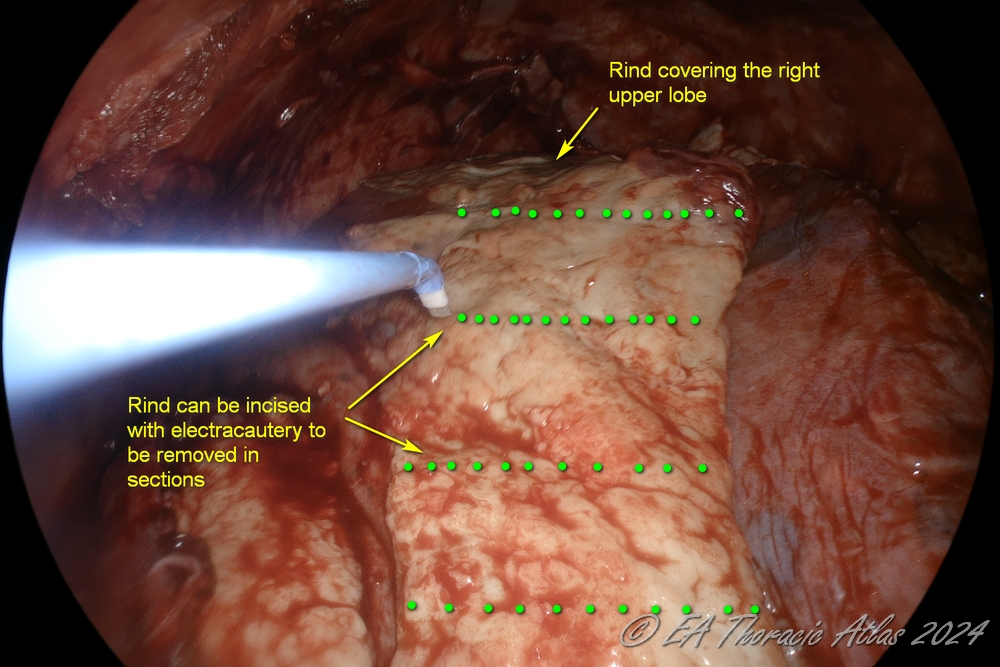
- Large areas of ring can be incised with electrocautery. Sections of rind then can be removed separately picking up the leading edge
- “Rolling” maneuver other the clapm may frequently help.
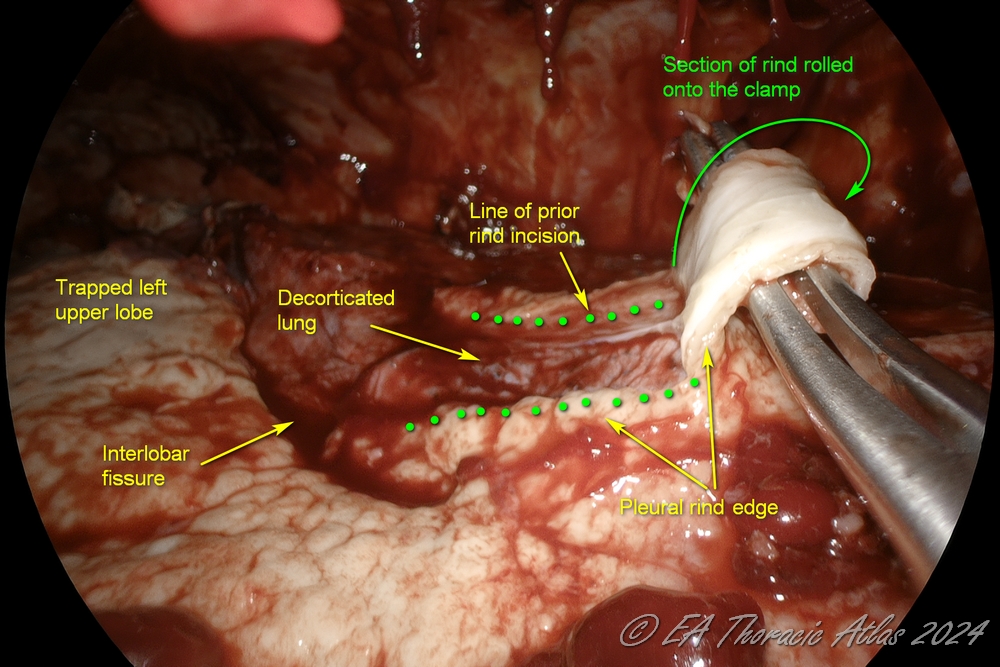
- Rarely peripheral subpleural abscesses that are encountered (usually can identified on the preoperative computed tomography imaging) They should be carefully unroofed, drained, and the wall biopsied to rule out malignancy. Postoperative air leaks from these areas are very uncommon.
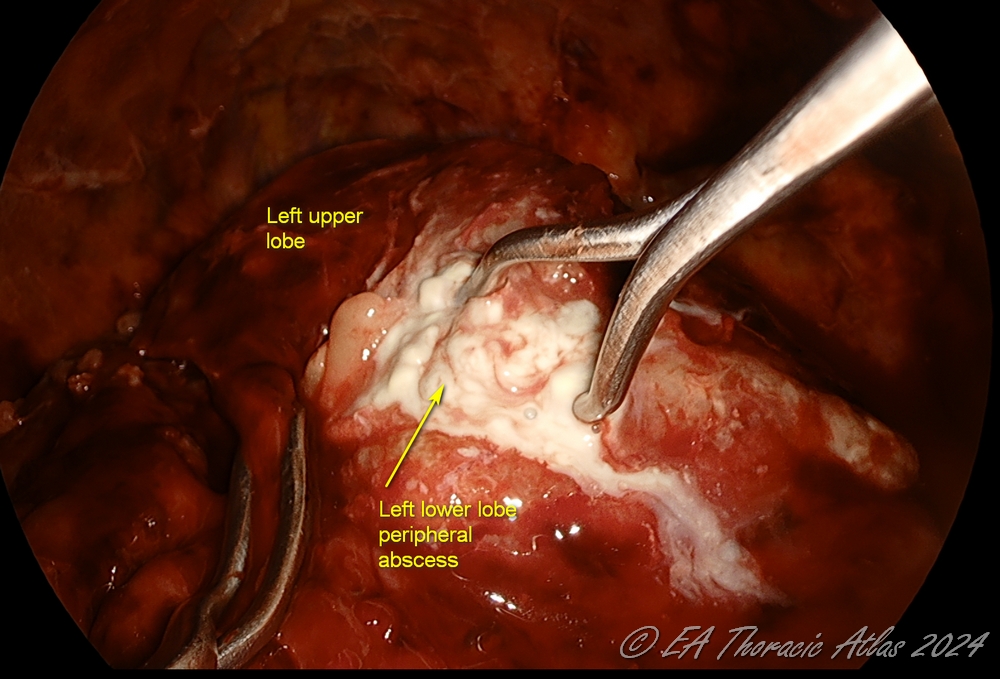
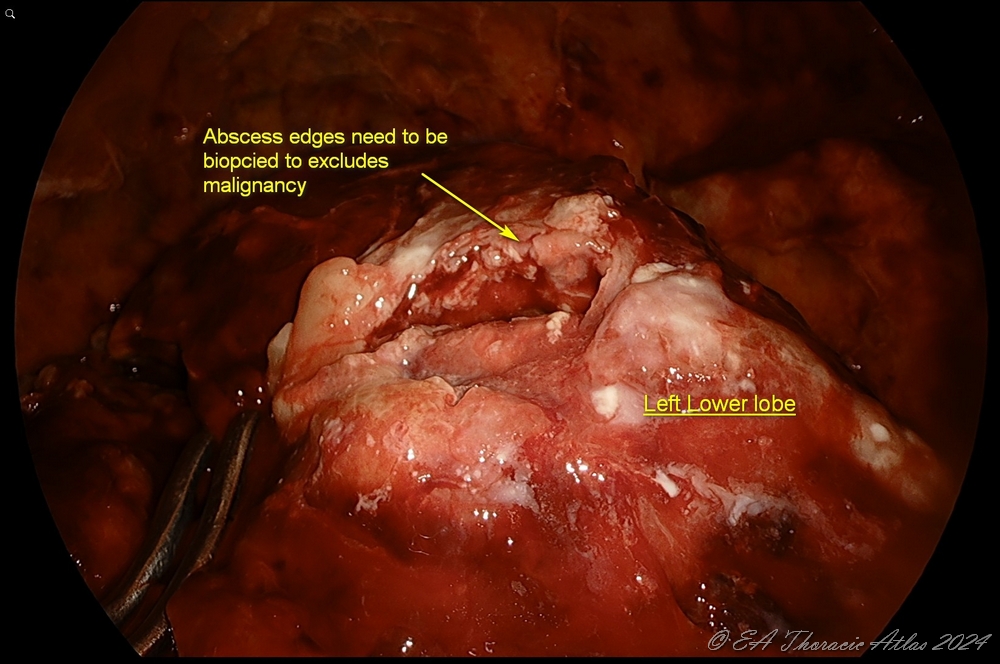
- Lung is reexpanded under direct vision to identify the areas that may need additional decortication.
- If the thoracoscopic approach is not feasible to perform complete decortication then convert to thoracotomy.
- There may be cases then the patient has very limited physiologic reserve and may not tolerate thoracotomy and potential blood loos. Partial decortication with addition of checkerboard pattern incisions to certain areas of the rind may improve lung expansion after empyema drainage. This should not be used liberally and reserved for selected cases only.
- One or two 32–28 Fr chest tubes are placed through the port sites. We frequently add addition side hole on the chest tube to maximize the draine area
- Straight—through the lower port and directed posteriorly towards the apex.
- Angled—through the upper port and directed between the lower lobe and diaphragm.
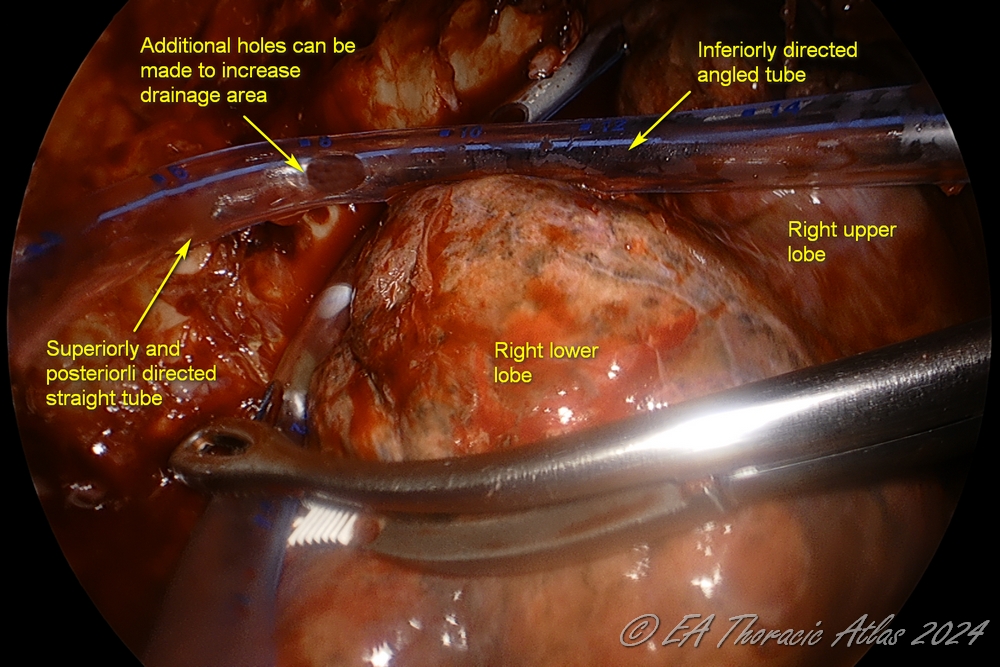
POSTOPERATIVE CARE
General
- Patients are extubated in the operating room, if appropriate, and admitted to the regular surgical ward.
- Some patients may develop septic response and are best admitted to the intensive care unit.
- Aggressive pulmonary care is essential.
- Ambulation at least four times daily.
- Scheduled bronchodilators and chest physical therapy with the use of a manual percussion device every 4 hours.
- Use mucolytics inhalers as needed.
- Incentive spirometer and PET therapy
Specifics
- Chest tubes are maintained on suction at –20 cm H2O for 2–3 days to facilitate maximal lung expansion and optimize drainage. Longer duration of suction may be needed if the lung does not stay expanded on water seal.
- Chest x-rays (CXRs) are used to monitor lung reexpansion (daily CXRs are not needed unless a change was made in the chest tube management or the patient’s condition changed).
- Chest tubes are removed after air leaks resolve and the output is <50 cc/day (for fibropurulent empyema). After the drainage of the parapneumonia effusion or the early exudate phase of an empyema, higher output (50-100cc/day) may be accepted for the removal.
- Small residual spaces may be observed after appropriate duration of antibacterial therapy.
- Patients with prolonged air leaks may be managed with outpatient chest tubes.
Outpatient follow up
- With adequately performed drainage/mobilization and decortication recurrence of effusion and empyema is very rare.
- CXRs should be performed 1–2 weeks after chest tube removal to assure lack of the effusion reaccumulation and full expansion of the lung. Routine follow up CT is not needed.
- Appropriate choice and duration of the antibacterial therapy needs to be established (usually inpatient Infectious Disease service consultation is obtained).