see: Thyroid nodule evaluation
see also: Thyroid Cancer (Evaluation and Management) and I131 sialadenitis (Radioiodine Sialadenitis)
and Parathyroidectomy
and Thyroid Hormone Replacement TSH free T4
see case example of surgery: Nonrecurrent recurrent laryngeal nerve
retrosternal anatomy: Mediastinal Tracheostomy with Anatomic Diagrams - Anatomy of Mediastinum - Clinical case example
see also: Retrosternal goiter (Substernal or Mediastinal Thyroid) - Sternotomy for Thyroidectomy
Note: last updated 2019
GENERAL CONSIDERATIONS
- Indications
- The indications for thyroidectomy or lobectomy vary from surgeon to surgeon and institution to institution. Local management practices, referral patterns, patient factors, and the reliability of cytologic evaluation combine to influence the treatment course adopted in many patients. The following is a compilation of indications for thyroidectomy or lobectomy used at this institution. It should be recognized that these indications may be modified based on the presumed pathology of the lesion and factors specific to the individual case. Consideration of all the factors influencing the decision to operate and which operation to do would be beyond the scope of this protocol outline (see Thyroid Cancer (Evaluation and Management) protocol).
- Diagnosis of malignant tumor of the thyroid by FNA or prior biopsy
- Vocal cord paralysis with an associated thyroid mass
- Palpable fixation of a thyroid mass to surrounding tissues
- Diagnosis of "follicular neoplasm" of the thyroid by FNA
- Diagnosis of "atypia of undetermined significance" a relative indication for surgery as of 2017 (Roman et. al, 2018)
- Single solid nodule greater than 3.0 cm
- Persistent reaccumulation of an apparent cystic mass despite aspirations or persistent aspiration of blood from an apparently cystic mass
- Symptoms of airway or esophageal compression with associated thyroid mass or goiter
- Patient desires to have a goiter removed for aesthetic reasons
- Patient desires to have a nodule removed regardless of presumed pathology
- As an adjunct to cervical esophageal surgery for improved access
- While not an absolute indication for thyroid surgery, a nodule present with a prior history of radiation to the neck strongly suggests an aggressive course of treatment
- Rapid growth of a solid thyroid mass
- Patient desires surgery rather than medical therapy or radioiodine treatment of Grave's disease
- A relative indication for thyroidectomy is the finding of metastatic thyroid disease in neck nodes without an obvious thyroid mass. The decision to perform thyroid surgery in this setting depends on the clinical situation under which the metastatic disease was found. If the metastatic disease was encountered in a palpable node in the absence of other head and neck cancer, thyroid surgery is indicated. When well-differentiated thyroid cancer is seen pathologically in a neck dissection specimen that also contains metastatic squamous cell cancer and there is no evidence of a thyroid mass, thyroidectomy is unlikely to alter the clinical course of the patient if radiation therapy is administered to the neck postoperatively. Thyroidectomy may be considered at a later date with sequential follow-up offered as an alternative employing ultrasound imaging.
- The indications for thyroidectomy or lobectomy vary from surgeon to surgeon and institution to institution. Local management practices, referral patterns, patient factors, and the reliability of cytologic evaluation combine to influence the treatment course adopted in many patients. The following is a compilation of indications for thyroidectomy or lobectomy used at this institution. It should be recognized that these indications may be modified based on the presumed pathology of the lesion and factors specific to the individual case. Consideration of all the factors influencing the decision to operate and which operation to do would be beyond the scope of this protocol outline (see Thyroid Cancer (Evaluation and Management) protocol).
- Contraindications
- Uncontrolled Grave's disease prior to medical stabilization of the patient.
- Riedel's thyroiditis: In this clinical situation simple division of the isthmus is recommended.
- Thyroidectomy in the face of anaplastic thyroid cancer may have a therapeutic effect if the lesion is small. However, in most instances, thyroid surgery is done to facilitate definitive airway management in these patients.
- Pertinent Anatomy
- The thyroid descends from the embryologic tongue from a point at the foramen cecum to rest in the anterior neck. The thyroid has two main lateral lobes. A midline pyramidal lobe is present in 50% of thyroids. The average weight of the adult thyroid is 20 to 30 grams.
- The thyroid is invested in a true capsule, which may only be separated from the parenchyma by sharp dissection. External to this, the thyroid is invested in the pretracheal fascia. The isthmus of the thyroid lies over the second and third tracheal rings.
- The most posterior extensions of the lateral lobes (tubercles of Zuckerkandl) are fixed to the trachea by Berry's ligament, which is a condensation of pretracheal fascia. This is considered the danger area for the recurrent laryngeal nerve. The recurrent nerve passes deep to Berry's ligament 75% of the time and through the ligament 25% of the time. In up to 10% of cases, the nerve may enter the true capsule of the thyroid for a short distance.
- The thyroid receives blood supply from paired superior and inferior thyroid arteries. The superior thyroid artery runs in close association with the external branch of the superior laryngeal nerve and therefore should be ligated as close to the thyroid capsule as possible to avoid injury to this nerve. The inferior thyroid arteries arise from thyrocervical trunks and pass posterior to the common carotid in their course to the thyroid. Although they are generally associated with the recurrent nerve, this association may be quite variable. The inferior thyroid artery generally provides blood supply to the parathyroid glands.
- The venous return consists of the superior, middle and inferior thyroid veins. The superior thyroid vein crosses the common carotid artery to empty into the internal jugular vein, and is the only thryoid vein to accompany an artery. The middle thyroid vein also crosses the common carotid to empty into the internal jugular. The inferior thyroid vein descends along the trachea to empty into the brachiocephalic veins.
- The right recurrent laryngeal nerve loops inferiorly around the subclavian artery, and tends to be slightly more lateral than the left, running in the tracheoesophageal groove in approximately 60% of cases. The left recurrent nerve loops around the aorta, and ascends maintaining a course more closely related to the tracheoesophageal groove in 70% of cases. A nonrecurrent, recurrent laryngeal nerve is much more common on the right and is always associated with a retroesophageal subclavian artery.
- The relationship of the distal segment of the RLN to the cricothyroid joint has been demonstrated to be a constant anatomical relationship. The right nerve coursed between 15 to 45 degrees 78% of the time, creating a more obtuse angle, while the left coursed between 0 and 30 degrees 77% of the time. (Otolaryngol Head Neck Surg. 2005 Oct;133(4):514-9.)
- The position of the superior parathyroids is variable but more constant than the inferior parathyroids (likely due to the shorter distance of migration of these glands during development). Approximately 80% of superior parathyroids will be located within 2cm of a point centered 1 cm above the intersection of the recurrent nerve and the inferior thyroid artery. These glands frequently lie in the paratracheal tissue and pretracheal fascia surrounding the gland, but may also be adherent to the true capsule of the thyroid. They are commonly found posterior to the RLN. The position of the inferior parathyroids is more variable, and about 60% will be inferior, lateral, or posterior to the lower pole. They are commonly found anterior to the RLN. The majority of the remaining glands are located inferiorly along the thyrothymic ligament or within the thymus.
PREOPERATIVE PREPARATION
- Evaluation
- The evaluation prior to thyroid surgery will largely depend on the indications for surgery.
- In general, patients being operated for a thyroid nodule should have a work-up including
- Complete exam of the head and neck including evaluation of vocal cord mobility, documentation of neck node status and size of thyroid nodule. The presence of a Chvostek sign should be evaluated preoperatively (approximately 5 to 10% of the normal population will have this sign with normal calcium levels).
- Fine needle aspiration
- Thyroid functions including TSH and free T4
- Endocrinology consultation
- Chest x-ray, with follow up imaging as indicated
- Potential Complications
- Hypocalcemia, which may be permanent see: Calcium Management in Thyroidectomy Patients - Hypocalcemia
- Vocal cord paralysis
- Postoperative hematoma or infection
- Transverse neck scar
- If a lobectomy is being performed for a neoplasm of undetermined pathology, the patient should be aware that completion thyroidectomy may be advised after final pathologic evaluation. Some patients will simply prefer to have a total thyroidectomy at the outset. This alternative and its potential risks should be detailed for the patient. The intraoperative plan should be detailed and decided upon with the patient prior to surgery. Potential modifications of the plan pending surgical findings and possible frozen section analysis of the nodule should also be discussed.
NURSING CONSIDERATIONS
- Room set up - see Basic Soft Tissue Room Setup
- Instrumentation and Equipment
- Standard
- Special
- Mahorner Thyroid Retractor Tray (available only)
- Tracheotomy Tray (available only)
- Maloney Esophageal Bougie Instrument Tray
- J/P bulb (250mL); fully-perforated 10cm Jackson-Pratt suction drain
- Gelpi self-retaining retractors
- Nerve stimulator control unit and instrument (Prass probe, Lingman or Parsons-McCabe)
- Medications (specific to nursing)
- Antibiotic ointment
- 1% lidocaine with 1:100,000 epinephrine
- Prep and Drape
- Standard prep using chlorhexidine solution
- Drape
- Head drape
- Square off with towels around neck, including chin, to 5 cm below clavicles laterally to trapezius muscles
- Split sheet
- Drains and Dressings
- Jackson-Pratt bulb drains, or Penrose drains (x2)
- Antibiotic ointment to suture lines (not applicable for Dermabond skin closure)
- Pressure dressing: Fluffs, Kerlix, burn netting
ANESTHESIA CONSIDERATIONS
- General
- Patient supine with shoulder roll
- May rotate 90 degrees (Pagedar, Bayon), along the long axis of the room (Sperry) or not rotate at all (Chang)
- Specific
- No paralysis
- Intraoperative RLN monitoring has become increasingly common, but is still not considered by all to represent the standard of care. Several studies have failed to show significantly lower rates of RLN paresis or paralysis using intraoperative monitoring. It is thought to be most useful in advanced stage or in reoperative situations. When performed, a special Laryngeal Monitoring Tube (LMT) tube is placed, with typically a 7.0 tube used for males, and 6.0 tube used for females. See Otolaryngol Head Neck Surg 2004 Nov;131(5):596-600 and Surgery 2004 Dec;136(6):1310-22. It important to consider that routine use of the nerve monitoring system provides valuabe experience and confidence in troubleshooting the problems that inevitably arise. To this end, the below troubleshooting guide has been developed using instructions provided by the device manufacturer’s guide (Medtronic NIM 3.0 Response), our own experience, and published algorithms (see Laryngoscope 2011 Jan;121 Suppl 1:S1-16. doi:10.1002/lary.21119)
-
Understanding the function of the nerve monitoring system as a circuit, as depicted in the included figure, is helpful in set-up and intraoperative troubleshooting. Figure legend: (A) Simple circuit shown with power source, including cathode and anode, from which current is derived, conducting wire, resistor or load, voltmeter, and ground. (B) Representation of the nerve monitoring system in its context as a circuit. Current is either purposefully generated with use of monopolar (e.g. Prass probe) or bipolar stimulating probes or unintentionally generated via electrocautery spread, nerve irritation through stretch, irrigation, or other noxious stimuli. The conductor in this circuit includes overlying fluids, fascia, muscle, fat, or other tissue, and the nerve itself, with current being transmitted at the neuromuscular junction as an electrical potential within the motor endplate. This results in vocalis muscle depolarization which is detected by the paired recording electrodes positioned on either side of the endotracheal tube (a pair of red electrodes positioned along the right vocalis muscle and another pair of blue electrodes on the left). The voltage difference between each recording cathode and anode pair is depicted as an EMG motor unit action potential on the nerve integrity monitor (NIM), left vocalis (blue) and right vocalis (red) respectively. Finally the remaining current is returned to the red and white return anode, usually placed in the subcutaneous tissues overlying the sternum, along with the green ground electrode.

-
Efficient troubleshooting of the nerve monitor is essential to its effective use. See the included algorithmic flow chart diagram for troubleshooting loss of signal events. Figure legend: When stimulus of a visible RLN does not result in signal, factors related to the patient and surgical field can be explored while simultaneously having operating room staff check the NIM setup before proceeding with additional troubleshooting as outlined. ETT – endotracheal tube, NIM – nerve integrity monitor.
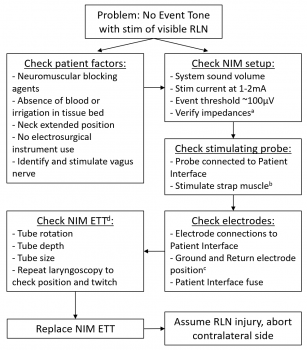
a Suggested <5kΩ on each channel, and <2kΩ difference between channels. If not, reposition tube, then replace if needed. If the neural monitoring unit indicates high impedance (>7.5kΩ), check the connection at the patient interface (white) box, consider repositioning the NIM ETT b For the stimulation probe, since output is pulsatile 4 per seconds, dragging over the tissue rather than hopping with the tip will give a more reliable result c Ground electrode should be placed in skin near bone without muscle (e.g. sternum), with system ground (green) closer to larynx than stimulus return (red/white), 5cm apart. d Avoid rotation of tube during intubation, as right-handed anesthesiologists tend to rotate the tube about 30 degrees clockwise inadvertently, typically requiring counterclockwise rotation typically for correction. Avoid tape on the lips as this tends to apply torque/rotational force that can displace the electrodes from optimal contact with the vocal cords. If needed, may use direct or video laryngoscopy to visually verify the ETT and evaluate for cord twitch with nerve stimulation. Verify no pooling of saliva which can result in salt bridging of current between electrodes. -
Additional complimentary troubleshooting steps should be considered for excessive noise in the system. See the included algorithmic flow chart diagram for troubleshooting excessive noise or continuous train signal events. Figure legend: When unexpected noise or continuous signal is elicited, begin with assessment of patient/surgical field factors along with the NIM setup with assistance from the circulating nurse before proceeding as outlined. ESU – electrosurgical cautery unit, ETT – endotracheal tube, NIM – nerve integrity monitor.
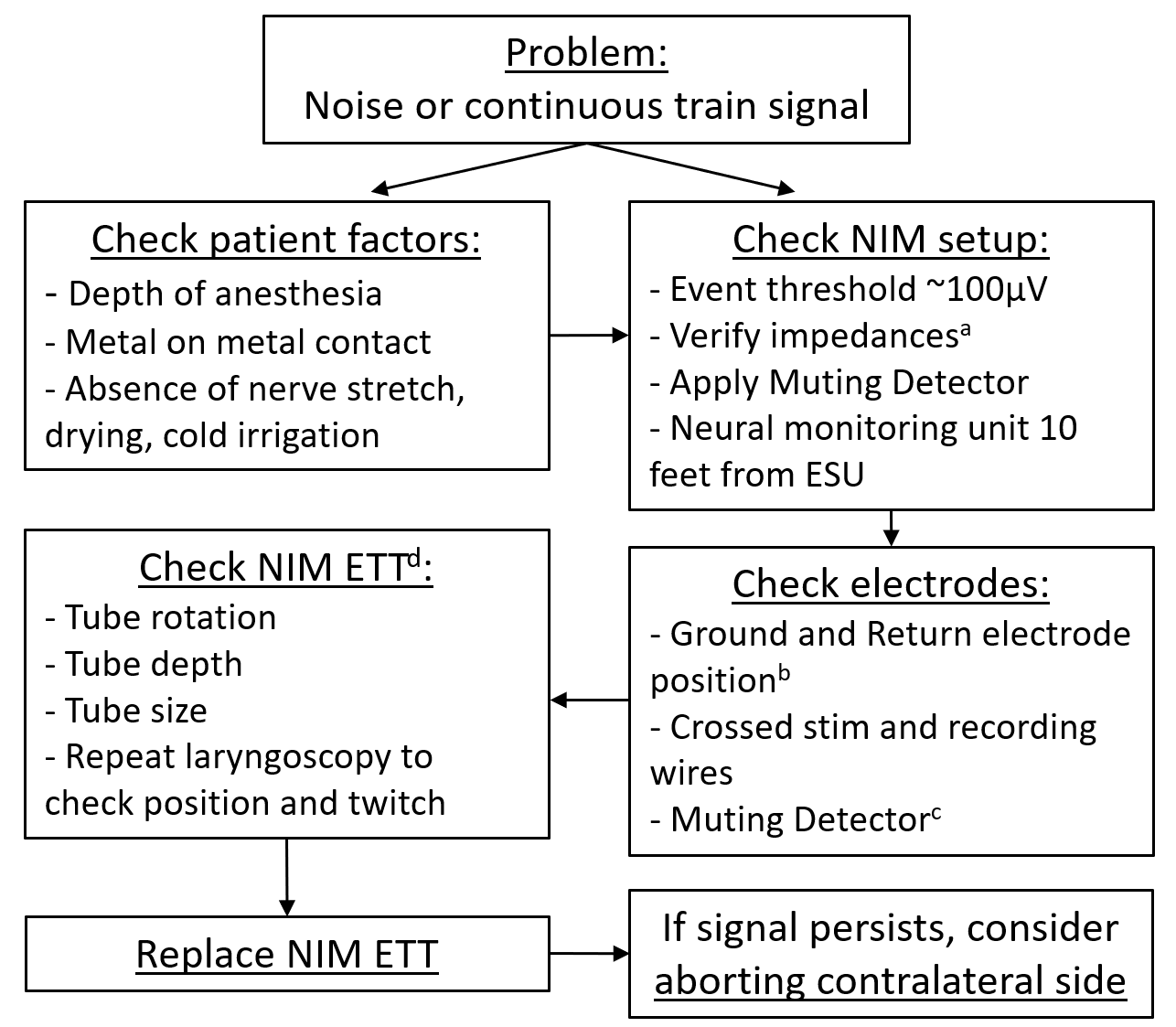
a Suggested <5kΩ on each channel, and <2kΩ difference between channels. If not, reposition tube, then replace if needed. Higher overall impedance bilaterally is preferred over large differential between sides as this leads to increased artifact and noise. If low impedance detection (<0.1kΩ), remove and replace with new NIM ETT. b Ground electrode should be placed in skin near bone without muscle (e.g. sternum), with system ground (green) closer to larynx than stimulus return (red/white), 5cm apart. Place ground and stimulus return on opposite side from pacer c Monopolar and bipolar cautery wires may be additionally looped through the Muting Detector for low electrosurgical unit settings. d If needed, may use direct or video laryngoscopy to visually verify the ETT and evaluate for cord twitch concurrent with unexpected signal. Verify no pooling of saliva which can result in salt bridging of current between electrodes.
-
OPERATIVE PROCEDURE
- Incision planning
- Incision should fall within a relaxed skin tension line between the clavicles and cricoid. The incision should be marked prior to extending the patients head as this may cause the incision to migrate over the clavicles post operatively resulting in a stretched scar.
- In females with large breasts, a slightly superior incision may ultimately result in a thinner scar.
- The textbook recommendation (Cummings, Meyer, Shah) is that the incision span from SCM to SCM. This does not always happen in practice. A good rule of thumb however is to review the preoperative imaging and know that the incision must be at least as large as the thyroid lobe you are removing. Also, a large incision will improve exposure, which will in turn allow for safer and less frustrating surgery.
- Once the incision has been marked, make an incision down to the subplatysmal plane. Alternatively, make an incision down to the strap muscles (Dr. Sperry's preference). It is imperative to maintain orientation when raising the skin flaps and also during separation of the strap muscles as the presence of a unilateral mass or an asymmetric goiter can significantly distort normal anatomic landmarks.
- Use 2-0 silk sutures for retraction of skin flaps. Alternatively, employ Gelpi, Mahorner or Alexis wound retractors to maintain separation of the skin flaps for exposure.
- After identification of the strap muscles and anatomic midline, the strap muscles should be separated and retracted.
- Separate strap muscles from capsule of thyroid.
- For total thyroidectomy, address the lobe containing the larger or more suspicious nodule first.
- Begin dissection by mobilization of superior pole. This is often greatly facilitated by division of some of the medial fibers of the sternothyroid muscle.
- Separate the avascular plane between the medial aspect of the upper pole and the cricothyroid muscle. This allows improved downward traction on the gland.
- Mobilize upper pole by dividing the branches of the superior thyroid artery and vein as close to the capsule of the gland as possible (this helps to protect the superior laryngeal nerve).
- After mobilization of the upper pole, retract the lobe medially and divide the middle thyroid vein if present. Staying in the plane between the true capsule and investing pretracheal fascia, mobilize the thyroid lobe medially until the cricothyroid joint is easily palpable. The joint is an excellent landmark for knowing where the recurrent nerve has to be.
- Apply lateral traction on the paratracheal tissues adjacent to the carotid and medial traction on the trachea. This expands the tissues within the tracheoesophageal groove and facilitates identification of the inferior thyroid artery and recurrent nerve.
- Gently spread orthogonal to the direction in which the nerve will be running with a hemostat within the expanded tissue to identify the nerve. While this may seem counterintuitive, if the hemostat is spread multiple times in the direction that the recurrent nerve runs, fibrous tissue will be aligned in that direction, and multiple tissue strands that "look like" the nerve will be created. In some patients, the nerve can be "palpated" or the outline of the nerve identified under fibrous tissue with gentle exploration using a Kitner.
- In most cases, the nerve is easiest to identify at the point where it has just emerged from the tracheoesophageal groove.
- Definitively identify the nerve by palpating the posterior cricoarytenoid muscle while stimulating the nerve. Movement of the muscle is easily palpated.
- Gently dissect the nerve to the point that it begins to turn posteriorly to run behind the cricothyroid joint. This is done with simultaneous mobilization of the thyroid lobe.
- The inferior pole is dissected and mobilized medially. As with the superior pole, branches of the inferior thyroid artery and vein are divided as close to the gland as possible.
- Final remaining attachments of Berry's ligament are divided. If the nerve is "knuckled" up medially in this area, the thyroid should be shaved from Berry's ligament using a knife, with hemostasis obtained using bipolar cautery, at a point safely medial to the nerve. When this is the anatomic configuration, it is not possible to remove 100% of the thyroid tissue without significant risk of nerve injury. However, the amount of thyroid tissue that needs to be left behind is small and should not result in more than a 0.5 to 1% uptake on the postoperative thyroid scan if obtained.
- A similar procedure is performed on the contralateral side if a total thyroidectomy is being performed.
- Before passing off the thyroid specimen, it should be carefully examined for the presence of parathyroids. If parathyroid tissue appears to be present, a small biopsy of it should be sent for frozen section. If it is confirmed to be parathyroid, the tissue should be divided into pieces approximately 1 x 1 mm and replanted into the SCM or muscle of the forearm.
- If a lobectomy and isthmusectomy is being done, the pyramidal lobe is dissected and removed, and the isthmus divided at the junction with the contralateral lobe.
- Meticulous hemostasis is obtained, and the dissected nerves are once again stimulated.
- The upper straps may be closed, but the straps are not reapproximated inferiorly
- Two 10 mm fully perforated suction drains are placed on either side of the wound and brought out through separate stab incisions in the neck.
- Closure
- 3-0 vicryl/polysorb or chromic to reapproximate strap muscles
- 3-0 monocryl or chromic to reapproximate the platysma
- 4-0 monocryl in a running subcuticular fashion followed by Dermabond. Alternatively, running 5-0 nylon may be used for skin closure
- A Queen Anne dressing or fluffs and burn netting are applied.
POSTOPERATIVE CARE
- The patient should have a postoperative check several hours after surgery and again in the evening by the on-call resident to exclude the presence of hematoma.
- See Calcium Management in Thyroidectomy Patients - Hypocalcemia
- The drains may be removed when drainage is below 30 cc per 24 hours. The Queen Anne dressing is removed in the following AM after surgery.
- If the patient will be having a postoperative thyroid scan, initial thyroid replacement is with Cytomel.
SAMPLE DICTATION
- A word of caution: The sample dictations below are not intended to be used as a template. They reflect only two of a multitude a variations on this procedure and should not under any circumstance substitute for the surgeon's own dictation. They are provided to help visualize the procedure from beginning to end and to illustrate key points and staff preferences.
- Informed consent was reviewed with the patient in the preoperative evaluation area. The patient was brought to the operating room and placed on the operating table in the supine position. The patient was then transorally intubated and an incision planned in a skin crease at 1 fingerbreadth above the clavicles. A shoulder roll was then placed. The neck was prepped with chlorhexidine and draped in sterile fashion. Lidocaine 1% and epinephrine 1:100,000 was injected in the incision line. A 15-blade knife was used to make the skin incision, which was carried down through the platysma to the level of the strap muscles. Subplatysmal flaps were not elevated to prevent hematoma. The strap muscles were split in the midline raphae. The thyroid isthmus was identified and split over the trachea. The left hemithyroidectomy was performed by dissecting strap muscles off of the nodule, and the superior pole of the thyroid gland was identified. In dissecting around the superior pole of thyroid, the superior parathyroid gland was identified and was dissected away from the thyroid lobe. The gland was then retract superiorly and laterally and the inferior pole was released. The thyroid lobe was retracted out of the surgical bed and the remaining attachments of the thyroid were released from the trachea. The thyroid was carefully examined, and there was no evidence of parathyroid tissue on it after removal. The left recurrent laryngeal nerve was identified and its identity verified with a nerve stimulator which resulting in contraction of the posterior cricoarytenoid muscle. Next, the right side was dissected free in a similar fashion. During dissection of the right lobe, the superior parathyroid gland and recurrent laryngeal nerve were also identified and spared. The right recurrent laryngeal nerve was able to be stimulated at the end of the procedure resulting in contraction of the posterior cricoarytenoid muscle. After both thyroid lobes were removed, all bleeding was stopped with bipolar cautery, and the wound was copiously irrigated with sterile saline. No evidence of any lymphadenopathy in level 6 was noted. A 15-French Jackson-Pratt drain was inserted and secured to the skin with 3-0 nylon suture. The wound was then closed by reapproximating the strap muscles and platysma with deep 3-0 Vicryl stitches. The skin was closed with a running 4-0 Monocryl subcuticular stitch and Benzoin and Steri-Strips placed over the incision. The patient tolerated the procedure well, was extubated in the operating room and transferred uneventfully to the post anesthesia care unit.
- Variation using a laryngeal nerve monitoring endotracheal tube (reflects Dr. Sperry's preference): Informed consent was reviewed with the patient in the preoperative evaluation area. The patient was brought to the operating room and placed on the operating table in the supine position. The patient was then transorally intubated with a laryngeal nerve monitoring endotracheal tube, with visualization to confirm correct placement of the electrodes. An incision was planned in a transverse skin crease below the cricoid, with the neck extended. The neck was prepped and draped in sterile fashion. An incision was made with the bovie, which was carried straight down through the platysma to the level of the strap muscles. The anterior jugular veins were identified and suture ligated. The fascia over the strap muscles was elevated superiorly and inferiorly, deep to the veins, and then these skin flaps were retracted widely. The strap muscles were split in the midline raphae. The thyroid tissue extending along the remnant thyroglossal duct tract was dissected first, removing this from the level of the hyoid back down to the isthmus. The left hemithyroidectomy was performed by dissecting strap muscles off of the nodule, and the superior pole of the thyroid gland was identified. In dissecting around the superior pole of thyroid, the superior parathyroid gland was identified and was dissected away from the thyroid lobe. The gland was then retract superiorly and laterally and the inferior pole was released. The thyroid lobe was retracted out of the surgical bed and the remaining attachments of the thyroid were released from the trachea. The thyroid was carefully examined, and there was no evidence of parathyroid tissue on it after removal. The left recurrent laryngeal nerve was identified and its identity verified with a nerve stimulator which resulting in contraction of the posterior cricoarytenoid muscle. Next, the right side was dissected free in a similar fashion. During dissection of the right lobe, the superior parathyroid gland and recurrent laryngeal nerve were also identified and spared. The right recurrent laryngeal nerve was able to be stimulated at the end of the procedure resulting in contraction of the posterior cricoarytenoid muscle. Informed consent was reviewed with the patient in the preoperative evaluation area. The patient was brought tothe operating room and placed on the operating table in the supine position. After both thyroid lobes were removed, all bleeding was stopped with bipolar cautery, and the wound was copiously irrigated with sterile saline. No evidence of any lymphadenopathy in level 6 was noted. A 15-French Jackson-Pratt drain was inserted and secured to the skin with 3-0 nylon suture. The wound was then closed by reapproximating the strap muscles and platysma with deep 3-0 Vicryl stitches. The skin was closed with a running 4-0 Monocryl subcuticular stitch and Benzoin and Steri-Strips placed over the incision. The patient tolerated the procedure well, was extubated in the operating room and transferred uneventfully to the post anesthesia care unit.
REFERENCES
Berry J. Suspensory ligaments of the thyroid gland. J Anat (London). 1888;22:IV-V.
Brauer RJ, Silver CE. Needle aspiration biopsy of thyroid nodules. Laryngoscope. 1984;94:38-41.
Halsted WS. The operative story of goiter. The authors operation. Johns Hopkins Rep. 1920;19:71-257.
Hoffman HT, Rojeski M, Funk GF, McCulloch TM. The solitary thyroid nodule. In Gates GA, ed. Current Therapy in Otolaryngology. St. Louis, Mo: Mosby: 1994:319-323.
Karlan MS, Catz B, Dunkelman D, Uyeda RY, Gleischman S. A safe technique for thyroidectomy with complete nerve dissection and parathyroid preservation. Head Neck. 1984;6:1014-1019.
Maceri DR, Babyak J, Ossakow SJ. Lateral neck mass sole presenting sign of metastatic thyroid cancer. Arch Otolaryngol Head Neck Surg. 1986;112:47-49.
Rush BF, Swaminathan AP, Patel R. A medial approach to thyroidectomy. Am J Surg. 1975;130:430-432.
Thompson NW, Brennan MF. Total thyroidectomy for cancer of the thyroid. In: Levensohn A, ed. Surgery Illustrated. New Scotland, New York: Learning Technologies Inc; 1985.
Vassilopoulou-Sellin R, Weber RS. Metastatic thyroid cancer as an incidental finding during neck dissection: significance and management. Head Neck. 1992;14:459-463.
Ward PH. The surgical treatment of thyroid cancer. Arch Otolaryngol Head Neck Surg. 1986;112:1204-1206.
Shindo M, Chheda NN. Incidence of vocal cord paralysis with and without recurrent laryngeal nerve monitoring during thyroidectomy. Arch Otolaryngol Head Neck Surg. 2007 May;133(5):481-5.
Shindo ML, Wu JC, Park EE. Surgical anatomy of the recurrent laryngeal nerve revisited. Otolaryngol Head Neck Surg. 2005 Oct;133(4):514-9.
Toll EC, Loizou P, Davis CR, Porter GC, Pothier DD.Scars and satisfaction: do smaller scars improve patient-reported outcome? Eur Arch Otorhinolaryngol. 2011 May 5. [Epub ahead of print]
Roman BR, Randolph GW, Kamani D. Conventional Thyroidectomy in the Treatment of Primary Thyroid Cancer. Endocrinol Metab Clin North Am. 2019 Mar;48(1):125-141. doi: 10.1016/j.ecl.2018.11.003. PMID: 30717897.