Novel Techniques for Renal Protection to enhance Novel Treatments for Neuroendocrine Tumors
Aim 1. Results from the pivotal NETTER-1 study clinical trial documented substantial improvement in progression-free survival with LUTATHERA® ([177Lu]-DOTATATE), leading to its approval in 2018 to treat adults with somatostatin receptor-positive neuroendocrine tumors. AZEDRA® (iobenguane I-131, [131I]MIBG) was also approved in 2018 for patients aged 3-12 years with MIBG scan positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma who require systemic anticancer therapy. AZEDRA® also demonstrates therapeutic potential for NETs. Despite improved overall survival with these individual therapies, the long term outlook for these patients remains poor. Individual efficacy of radionuclide-based cancer therapies is ultimately limited by damage to internal normal organs from the radiation dose to these agents. Both the targeting mechanism as well as the dose limiting organs are distinctly different for AZEDRA® when compared to LUTATHERA®. Earlier studies have demonstrated the therapeutic value of [131I]MIBG in the subset of MIBG-positive midgut NETs (50-60% of patients) and more recent studies found notable survival benefits with [131I]MIBG treatment for metastatic neuroendocrine tumors. This proposed clinical trial is based on our novel concept which combines LUTATHERA® and AZEDRA® resulting in increased therapeutic radiation dose to tumors without exceeding radiation exposure limits to dose limiting organs by adding a specifically and individually calculated amount of AZEDRA® to LUTATHERA®. This clinical trial has the potential to provide durable therapeutic benefit for hundreds of patients where other therapeutic strategies fall short.
General trial design. SOC: Standard of Care care
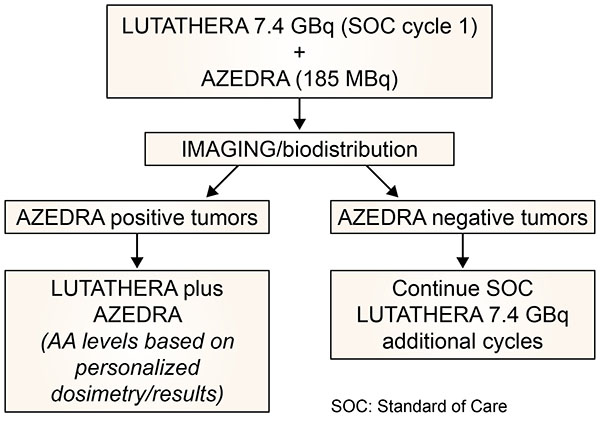
Schema of proposed trial
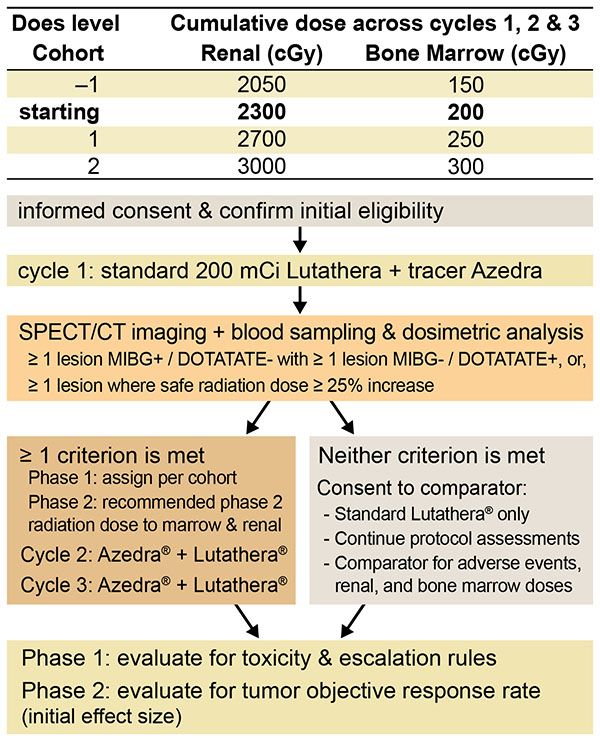
Aim 2.Partial reabsorption of radiopeptides in kidneys limits the dose that can be delivered to tumors, because residual radiation in kidneys can lead to increased reactive oxygen species (ROS) production and oxidative stress in glomeruli and proximal tubules that can lead to injury. ROS mediate tubular fibrosis that can impair kidney function. Nanozymes can be designed to deliver active-antioxidant enzymes (e.g., superoxide dismutase; SOD) and competitive inhibitors (e.g., Lys residues) specifically to kidneys that can reduce radiopeptide reabsorption in kidneys and reduce the adverse sequelae that limit radiation doses. Successful implementation of this technology has the potential to result in an approach that transforms receptor targeted radionuclide therapy by allowing higher levels of radiation to be delivered to tumors, while minimizing kidney injury risk.
[203Pb]DOTATOC uptake concentrates in somatostatin receptor positive tumors as well as in kidneys and bladder
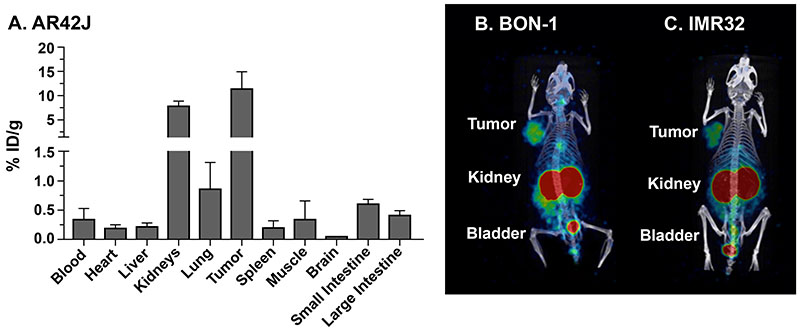
Biodistribution and [203Pb]DOTATOC SPECT/CT imaging of SST2R positive tumors in mice. (A) Biodistribution of [68Ga]DOTATOC in mice bearing human AR42J (high SST2R) at 1.5 h post injection (0.37 MBq; 10 mCi); (B-C) Mice bearing BON-1 (low SST2R) and IMR-32 (high SST2R) tumors were imaged by SPECT/CT at 2 h post injection of 6.7 MBq (180 mCi) [203Pb]DOTATOC. These data demonstrate that the primary off target organ accumulation of SST2R targeted peptides (octreotide based) is the kidneys. These data further show that the AR42J model is an effective model for our biodistribution studies.
[212Pb]DOTATOC prolongs survival of mice with neuroendocrine tumor, but also leads to kidney damage
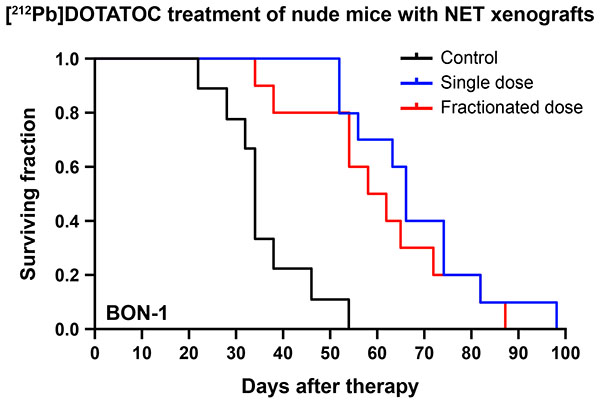
Survival curves after [212Pb]DOTATOC treatment. Mice bearing BON-1 tumors expressing somatostatin receptors were divided into three groups (Control, 120 mCi single dose, and 3 fractionated doses of 50 mCi, 50 mCi, and 20 mCi). [212Pb]DOTATOC was injected via tail vein (n=9 for control, n=10 for the other groups). Mice were euthanized when tumors reached 1500 mm3. These data support the hypothesis that 212Pb will produce objective tumor responses to [212Pb]PSC-TOC in patients with SST2R+ tumors.
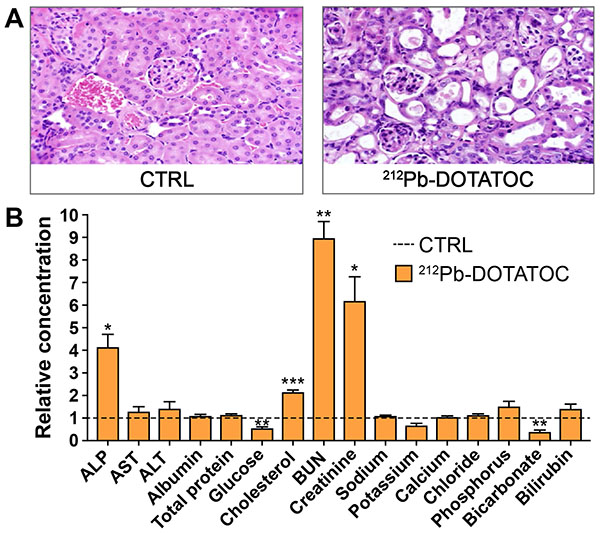
High doses of radionuclide therapy can lead to significant kidney damage. (A) Kidney histopathology after 6 months post-injection of 120 µCi [212Pb]DOTATOC in C57BL/6 mice. (n=3) (B) Blood chemistry 6 months post-injection of 120 µCi of 212Pb-DOTATOC in C57BL/6 mice. Data expressed relative to control. *P<0.05, **P<0.01, ***P<0.005. These results provide a rationale for conducting the proposed research to improve outcomes for NET patients.
The goal of this research is to use nanozymes containing Superoxide Dismutase to protect kidneys from reactive oxygen species
Composition of Nanozymes
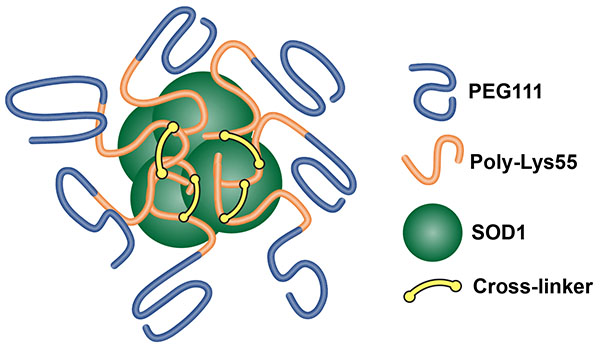
Illustration of self-assembled cross-linked nanozymes encapsulating active SOD1 used for gathering preliminary data shown in this proposal. Cross-linker is composed of 3,3'-dithiobis(sulfosuccinimidyl propionate): MW 120 kDa; in vivo half life ~30 min; size ~33.9 nm dia.
Nanozymes can be targeted to Kidneys
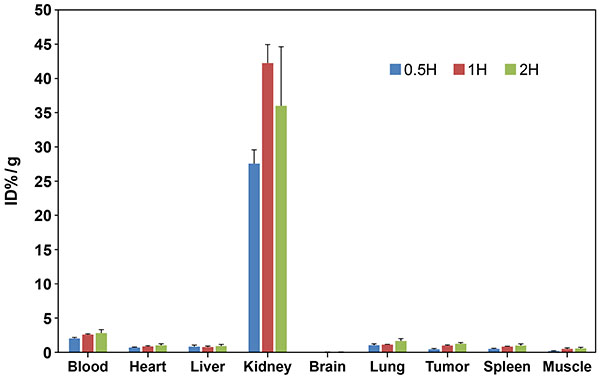
In vivo biodistribution of 125I-SOD1-nanozymes in melanoma tumor bearing mice. 125I-SOD1-nanozymes were injected via tail vein (n=3). Tumors and organs of interest were harvested at 0.5h, 1h and 2h. Data is normalized to percent injected dose per gram of tissue (ID%/g) ± SEM. These data support the hypothesis that nanozymes can be designed to selectively deliver active antioxidant enzymes and competitive-inhibitors to kidneys.