More effective therapies to treat advanced, metastatic NETs
Meaningful biomarkers and effective therapies are urgently needed for patients with advanced, metastatic neuroendocrine tumors (NETs). Project 2 employs hypothesis-driven investigations of tumor promoting pathways to develop new NET therapeutics through an integrated ‘omics’ approach. The goal is to identify new biomarkers, drug targets and therapies for metastatic NETs. Biomarkers and new drugs will be validated in powerful preclinical models of NET metastasis, including patient-derived tumor organoids. The biomarkers are expected to guide future clinical trials of novel combination therapies and to serve as prognostic biomarkers of patient outcomes that will improve the clinical management of patients with metastatic NET disease.
Our team is led by Drs. Dawn Quelle (PhD), James Howe (MD) and Ben Darbro (MD, PhD). Significant advances (see recent manuscripts) have been made in the following three areas:
- Establishing inexpensive, chromosome-based biomarker tests for NETs
- Identifying more effective targeted therapies for advanced NETs
- Developing new NET model systems for rapid testing of therapies
Chromosome based biomarkers
- Examining 30 unique tissue microarrays (TMAs)
- 470 sbNET tissue samples from >110 patients
- 202 pNET tissue samples from >70 patients
- Each tissue sample represented in triplicate on the TMA
- 100 disease progression events
- 70 death events
- ~100 nuclei scored per FISH probe
- Five FISH probe sets to score
- Potentially >1 million nuclei to score
- Evaluating each probe for gain, loss, or wildtype status for both site of NET origin and prognosis
- Develop clinical kits for rapid (~48-72 hr), relatively inexpensive patient tumor analyses
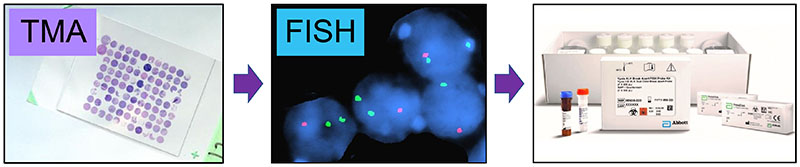
Figure 1. Workflow of developing chromosome-based biomarkers kits for identifying NET site of origin and predicting patient outcomes.
More effective targeted therapies
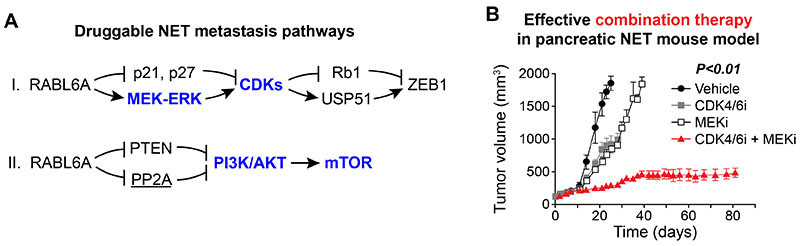
Figure 2. Oncogenic RABL6A acts through druggable pathways with known roles in tumor metastasis.
A. Overview of the two major RABL6A activated pathways in metastatic NETs. Bolded factors in blue are targets for FDA-approved inhibitors used clinically; underlined PP2A, re-activating drugs are in development by our University of Michigan collaborator, Dr. Goutham Narla.
B. Targeting MEK-CDK pathway in pancreatic NETs. Daily treatment by oral gavage of pancreatic NET xenograft tumors (10 mice per group) with a MEK inhibitor (MEKi, 3 mg/kg) plus CDK4/6 inhibitor (CDK4/6i, 12.5 mg/kg) had significant synergy and caused sustained inhibition of tumor progression. P<0.01 for comparison of this novel combination therapy (red) versus each drug alone or vehicle control.
New NET models and drug testing

Figure 3: PNET and SBNET patient-derived organoids (PDOs) characterization and drug testing. A) Microscopy images of PNET PDOs stained with specific antibodies against NET markers: synatophysin (SYP) and Chromagranin A (CHGA) and Somatostatin Receptor 2 (SSTR2). B) Protein expression levels of NET markers in PNET PDOs and control cell line (HepG2). C) Area of PNET PDOs in culture with respect to time. D) Drug sensitivity of PNET PDOs cultures to drug treatment. E) Microscopy images of SBNET PDOs stained with SYP, CHGA, and SSTR2. F) Protein levels of NET markers in SBNET PDOs and control PANC-1 cells. G) Area of SBNET PDOs in culture with respect to time. H) Drug sensitivity of SBNET PDOs. Data are represented as the mean ± standard error of the mean.