Stress changes a structural protein into a gene regulator that protects against heart failure
An unexpected finding that links a structural heart protein to gene regulation following heart stress suggests potential new avenues for developing heart failure therapies.
The work, led by University of Iowa heart researcher Long-Sheng Song, MD, focuses on a protein called junctophilin-2 (JP2). Previous work from Song’s lab has shown that JP2 is a structural protein that is essential for heartbeat and that loss or disruption of JP2 is associated with heart failure.
Study published Nov. 8

“We have long known that this protein is a structural protein, important for muscle function, but we never expected it to also have this ability to regulate gene expression,” says Song, professor of internal medicine at the UI Carver College of Medicine. “These findings reveal a previously unknown self-protective mechanism that heart muscle cells possess to counter the damaging effects following cardiac stress.”
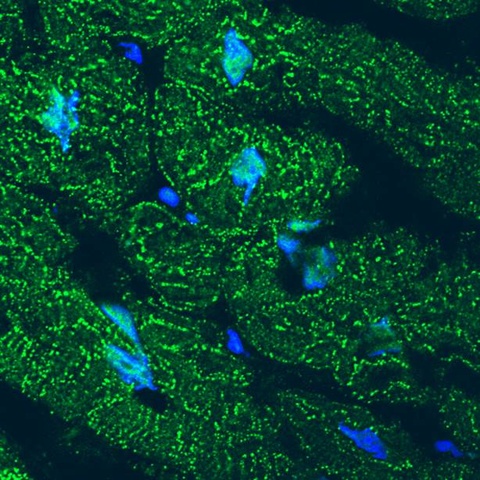
A University of Iowa study finds that cardiac stress transforms an important structural protein into a gene regulator that protects against heart failure. The image shows accumulation of the N-terminal fragment of the protein junctophilin-2 (JP2) in the nuclei of failing human heart muscle cells. Green shows the location of JP2 or its N-terminal fragment. Blue indicates the cell nuclei. Under stress conditions, JP2 is cleaved into two fragments. The cells’ membrane structure is severely damaged due to loss of the full length JP2. But the JP2 fragment migrates to the cell nuclei and turns off genes that promote heart failure. Image courtesy of Long-Sheng Song, University of Iowa.
Heart disease conditions—like high blood pressure, blocked arteries, or heart attack—all put stress on the heart. At the cellular level, this type of stress activates an enzyme that cleaves JP2 into two fragments. The new study shows that the N-terminal JP2 fragment migrates to heart cell nuclei and initiates genetic changes that protect against heart failure. The DNA sequences that allow the fragment to travel to the nucleus and that regulate gene expression are highly conserved among many species from mice to humans.
To prove the beneficial effect of the JP2 fragment, the researchers engineered mice with increased levels of the JP2 N-terminal fragment. These animals were protected from developing heart failure in response to cardiac stress. Conversely, mice genetically engineered to lose the function of the JP2 fragment in the nuclei developed heart failure at an accelerated rate following cardiac stress.
Findings hint at possible heart failure treatment
“Our findings suggest that increasing the level of JP2 fragment or functional peptide in the heart might hold promise as a strategy for treating heart failure,” says Song, who also is a member of the Fraternal Order of Eagles Diabetes Research Center at the UI and holds a staff appointment with the Iowa City Veterans Affairs Medical Center. “We have secured a patent for the use of the protein fragment, and intend to investigate gene therapy approaches for delivering it to heart cells in preclinical (animal) models of heart failure.”
In addition to its role in heart muscle, JP2 is also important and abundant in other types of muscle (skeletal and smooth). The new findings suggest that JP2 fragmentation may play a role to protect against the adverse effects of stress in all types of muscle.
Members of the research team
Song’s colleagues on the study included Ang Guo, PhD, UI research assistant professor in internal medicine, who was the first author of the study, and UI researchers Yihui Wang, Biyi Chen, Yunhao Wang, Jinxiang Yuan, Liyang Zhang, Duane Hall, Jennifer Wu, Yun Shi, Qi Zhu, Cheng Chen, William Thiel, Xin Zhan, Robert Weiss, Fenghuang Zhan, Catherine Musselman, Miles Pufall, Kin Fai Au, and Chad Grueter from the Abboud Cardiovascular Research Center, and the Departments of Internal Medicine and Biochemistry at the UI Carver College of Medicine. The research team also included Mark E. Anderson at Johns Hopkins School of Medicine; Jiang Hong at Shanghai Jiao Tong University School of Medicine; and Weizhong Zhu at Nantong University, China.
This work was funded by grants from the National Institutes of Health, the Department of Veterans Affairs, and the American Heart Association.