A small drug molecule that’s already approved to treat fungal diseases can correct lung and airway problems caused by cystic fibrosis (CF) by restoring key defense mechanisms against infections that are disrupted by CF. The drug molecule operates as a “molecular prosthesis,” substituting for the protein channel that is missing or defective in the lungs of people with CF.
The new findings, which were published March 13 in the journal Nature, come from a collaborative research study led by Martin Burke, MD, PhD, at University of Illinois at Urbana-Champaign, and Michael Welsh, MD, at the University of Iowa Carver College of Medicine.
Study background
Cystic fibrosis is a lifelong genetic disease that makes patients vulnerable to lung infections. Several new drugs approved within the past seven years provide effective treatments for some but not all patients, and there is still no cure.
The Illinois-Iowa team tested a drug called amphotericin and showed that it restored infection-fighting properties in lung tissue donated by human patients as well as in pigs with CF. The drug has the potential to become the first treatment to address all types of cystic fibrosis, regardless of the genetic mutation that causes the protein deficiency.
“Instead of trying to do gene therapy—which is not yet effective in the lung—or to correct the protein, our approach is different. We use a small molecule surrogate that can perform the channel function of the missing protein, which we call a molecular prosthetic,” says Burke, a professor of chemistryat Illinois and associate dean for research at the Carle Illinois College of Medicine.

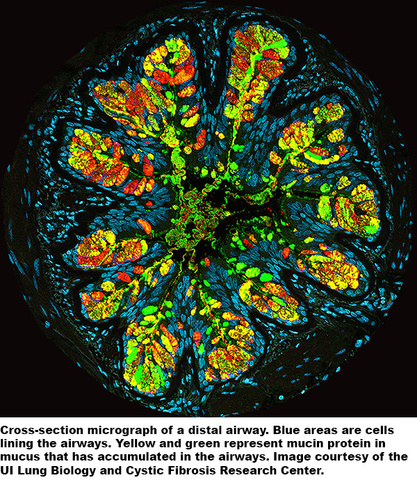
“Losing CFTR channel function makes airway surface liquid more acidic and disrupts salt secretion. These defects cripple two important lung defenses: the antibiotic activity of airway liquid and the clearance of mucus. As a result, people become vulnerable to infection,” says Welsh, UI professor of internal medicine and a Howard Hughes Medical Institute investigator.
CF milestones at Iowa
Since 1985, Iowa’s cystic fibrosis research has yielded a number of important discoveries. Read more.
Studying features of amphotericin
Burke’s group has a long-standing interest in the channel-forming properties of amphotericin (am-foe-TARE-is-in), a drug used to treat fungal infections. In the new study, the researchers explored its potential as a treatment candidate for CF. They found that amphotericin can form channels in the surface membrane of lung tissue donated by people with CF that was caused by various mutations in the CFTR gene. The channels released bicarbonate and brought the acidity and viscosity of the airway surface liquid back within normal range.
The researchers also used a version of amphotericin formulated for delivery to the lungs to treat pigs with CF. In both the experiments, in human tissue and in pigs, the researchers saw a restoration of the infection-fighting properties in the liquid lining the lung surfaces.
The molecular prosthetic
“Just as a simple prosthetic device can restore a lot of function to those missing a limb, we found that although amphotericin is not a perfect mimic of the CFTR protein, it can function as a bicarbonate channel and restore defense mechanisms in the airway surface liquid,” Burke says.
Unlike the current CF drugs that target defective CFTR proteins and work to correct their misfolded structure, the molecular prosthetics approach bypasses the defective protein to form new channels—an important feature for the 10 percent of people with CF who are completely missing the CFTR protein and therefore cannot be treated with corrector drugs.
“Whereas many of the latest advances in cystic fibrosis treatment have been targeted to specific mutations, this approach would benefit everyone with cystic fibrosis, regardless of mutation,” says Emily Kramer-Golinkoff, a co-founder of the nonprofit cystic fibrosis research foundation Emily’s Entourage, which in part funded the research. “Second, and perhaps even more importantly, this approach presents an opportunity to repurpose an existing, approved drug and bring it to the clinic quickly.”
Future steps
Next, the joint Illinois-Iowa research team hopes to conduct clinical trials to see whether amphotericin delivered to the lungs is effective in humans with CF.
“The cystic fibrosis community is truly in need of new therapies to reduce the burden of this disease. We are interested to see how this potential treatment performs in clinical trials in the future,” says James Kiley, director of the Division of Lung Diseases at the National Heart, Lung, and Blood Institute, part of the National Institutes of Health, which also funded the work.