University of Iowa Carver College of Medicine researchers, in collaboration with Belgian scientists, have shed light on a new host defense mechanism that targets viruses and intracellular bacterial pathogens.

In order to evade “first-responder” immune responses, certain viruses and bacteria hide inside cells. However, there are intracellular immune mechanisms that can counteract these pathogens. The laboratory of Lilliana Radoshevich, PhD, UI assistant professor of microbiology and immunology, studies intracellular host defense mechanisms, specifically focusing on the role of ISG15, a protein that “tags” other proteins. Previous studies have shown that ISG15 is involved in clearing viral, bacterial, and parasitic infections, including influenza A/B virus, Mycobacterium tuberculosis and Toxoplasma gondii.
But despite its broad antimicrobial role, how ISG15 functions at a molecular level is not well understood.
In the new study, published recently in Nature Communications, the research team used a new technique—developed by Francis Impens, PhD, and colleagues at the VIB-UGent Center for Medical Biotechnology in Belgium—to trap and identify proteins that interact with ISG15 in order to better understand its function. Using this method, called “virotrap,” they uncovered an interaction between ISG15 and a mega-protein called Ring Finger Protein 213 (RNF213). Very little is known about RNF213, but mutations in this protein result in Moyamoya disease, a rare disorder that causes patients to have strokes at a young age.
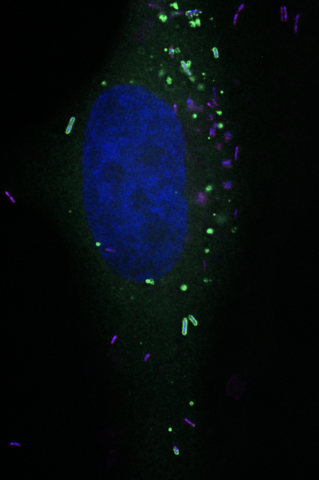
RNF213 is also known to stabilize lipid droplets in the cell. Long thought of as simple energy depots, lipid droplets have recently been shown to play an antimicrobial role. The new study finds that on the surface of lipid droplets, RNF213 functions as a sensor for ISG15-tagged proteins. Strikingly, not only does RNF213 localize to the surface of lipid droplets, but it also docks onto intracellular invading Listeria monocytogenes.
Experiments that removed or increased the RNF213 protein in cells and in animal models showed that it has antibacterial activity against Listeria monocytogenes and other pathogens, and revealed this new role for RNF213 in antimicrobial defense.
“It was particularly exciting when we first observed RNF213 perched on intracellular bacteria,” says Radoshevich. “Linking RNF213 to intracellular clearance of viruses and bacteria may also help us understand and unlock the mysterious etiology of Moyamoya disease.”
The study was funded in part by an R35 MIRA grant from the National Institute of General Medical Sciences.
Read the study
From Nature Communications, Oct. 1, 2021: "Ring finger protein 213 assembles into a sensor for ISGylated proteins with antimicrobial activity"