A University of Iowa study has identified a method for inhibiting the occurrence of an aggressive form of arthritis that frequently develops following a severe traumatic injury.
The preclinical work demonstrates the potential for preventing posttraumatic osteoarthritis (PTOA) by targeting very early damage after fractures in an animal model of this type of injury. These types of fractures in a load-bearing joint can lead to arthritis as quickly as two years after injury.
Background on the condition
PTOA is present in some 5.6 million people in the United States. The disease occurs when cartilage is destroyed in a joint, leading to life-long pain and disability. The young and active patients who are disproportionately likely to experience these injuries are not good candidates for joint replacements, so preventing PTOA in this group is a particularly compelling need.
“The time to complete joint destruction can be as fast as two to four years,” says the study’s corresponding author, Mitchell Coleman, PhD, research assistant professor of orthopedics and rehabilitation in the UI Carver College of Medicine. “That can be devastating for an 18-year-old who injures an ankle falling off a ladder or playing sports.”


At left, Mitchell Coleman, PhD, and James Martin, PhD
Researchers focus on healthy cartilage cells
Previous work has shown that relatively few chondrocytes—the cells that make up healthy cartilage tissue—are killed at the moment of impact when a joint is fractured. Instead, cell death increases in the 48 hours following the injury, suggesting that biologic activity unleashed by the impact may contribute to early causes of disease.
The University of Iowa study, published Feb. 7 in Science Translational Medicine, focused on reducing oxidative stress in the mitochondria of chondrocytes after injury. Mitochondria are organelles that serve as the powerplant of a cell and are also an important biological source of oxidants. During normal mitochondrial function, only small, relatively innocuous concentrations of oxidants are produced.
A history of work in mitochondrial metabolism
The lab of James Martin, PhD—senior author of the study and UI associate professor of orthopedics and rehabilitation and biomedical engineering—has a long-standing research focus on severe mechanical injuries that lead to intense increases in mitochondrial metabolism. These increases result in larger, more damaging concentrations of oxidants and oxidative damage, capable of contributing to rapid PTOA development.
Using a pig model of ankle fracture, the researchers employed two approaches to limit oxidative damage in the chondrocytes: inhibiting mitochondrial metabolism with amobarbital, and boosting antioxidants in the chondrocytes with N-acetylcysteine (NAC).
Results suggest a possible course of acute treatment
“We demonstrated that the posttraumatic osteoarthritis that occurs in an ankle after a severe injury can be significantly blunted by inhibiting mitochondrial metabolism or adding key antioxidants immediately after injury,” Coleman says. “These treatments were only given twice, once right after injury and once a week later. No chronic therapy was used. Our data suggests that there might be a way to treat people acutely after they break their ankle to prevent PTOA.”
By using a pig model instead of the customary mouse model, the researchers were able to closely mimic the treatment a person would receive following a traumatic ankle fracture and test the safety and effectiveness of the treatment in a large animal model. This approach enhances the translational possibilities of the researchers’ work.
The group has applied for grant support to conduct preliminary clinical trials of the approach at multiple institutions.
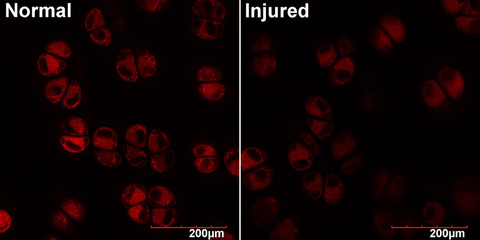
Mechanical impacts like those associated with intraarticular fractures deplete chondrocyte mitochondria
High magnification, 100X objective images of animal articular cartilage show that chondrocytes subjected to injury stain less brightly for mitochondrial indicators like Mitotracker Deep red, shown here. This defect in intracellular metabolism persists after injury, and the researchers believe it is a significant contributor to posttraumatic osteoarthritis.
The research team
In addition to Coleman and Martin, the team included UI researchers Jessica Goetz, Marc Brouillette, Dong Rim Seol, Michael Willey, Emily Petersen, Nathan Hendrickson, Jocelyn Compton, Behnoush Khorsand, Angie Morris, Aliasger Salem, and Douglas Fredericks. The team also included Todd McKinley from Indiana University and Hope Anderson from Wellesley College.
The research was supported in part by funding from the U.S. Department of Defense and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Several of the research team members, including Coleman and Martin, are inventors on a patent held by the University of Iowa Research Foundation that covers the use of the drug loaded- reverse thermal hydrogels for prevention of PTOA. The patent is exclusively licensed to CartilaGen Co., a University of Iowa spin-off company developing innovative treatments for osteoarthritis. Salem serves on the advisory board of CartilaGen Co., and Martin serves as scientific adviser to the company.