Return to: Granulomatosis with Polyangiitis or Subglottic Stenosis - Example Cases
see also: Spirometry PIF Peak Inspiratory Flow; Treatment of sublgottic stenosis with sialoballoon salivary balloon dilation
CLINICAL HISTORY
A 31 year old female presented to UIHC on 8/13/2010 with a history of facial fullness starting in the fall of 2009, prior to which she had been healthy. She was treated with antibiotics for sinusitis at the time with questionable improvement. In February 2010, she started to develop intermittently blood-tinged nasal discharge, and she received another course of antibiotics with no improvement. She then developed progressive hoarseness and loss of voice, and in July 2010 she started to experience shortness of breath and stridor.
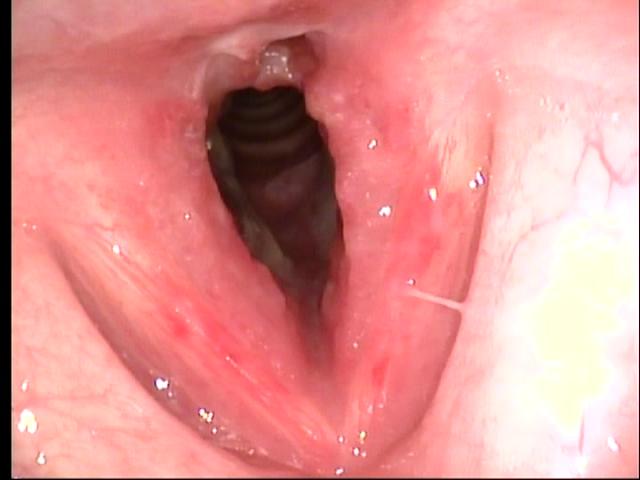
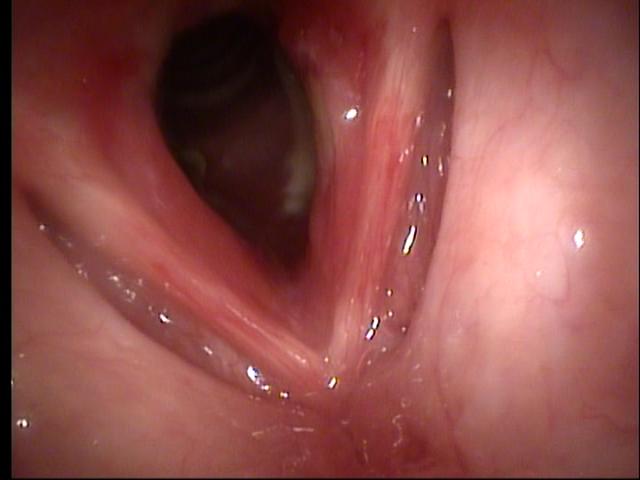
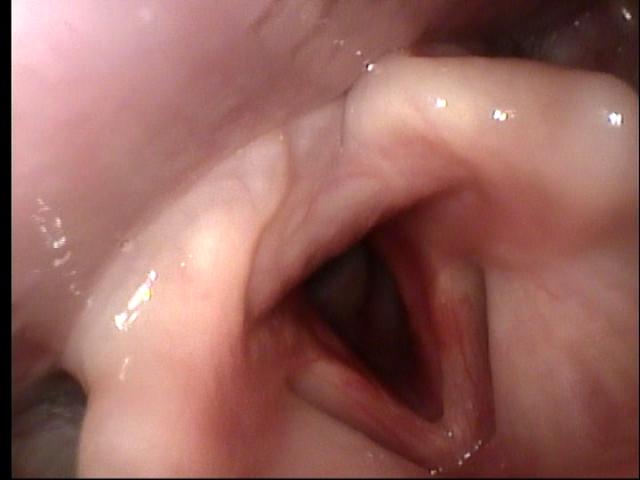
Sequence of transnasal laryngoscopy images following initial presentation (click to enlarge and hover over right middle edge to advance with arrows).
 |
 |
 |
|
Transnasal fiberoptic laryngoscopy at UIHC 08/13/10 (day 4 of prednisone)
|
Transnasal fiberoptic laryngoscopy at UIHC 08/17/10 (day 8 of prednisone)
|
Transnasal fiberoptic laryngoscopy at UIHC 08/31/10 (day 22 of prednisone)
|
DIAGNOSIS, DISEASE COURSE, AND TREATMENT
- Jul 2010 - Patient undergoes single course of prednisone 20 mg daily; evaluated by local rheumatologist with concern for relapsing polychondritis vs. Wegener's granulomatosis, though ANCA screen was negative at the time
- 8 Aug 2010 - evaluated by local otolaryngologist for worsening airway status, who suggests tracheotomy, though she opts instead for high dose steroids (prednisone 40 mg bid)
- 13 Aug 2010 - evaluated by otolaryngology at UIHC; flexible laryngoscopy shows intra-arytenoid scarring and subglottic granulation tissue; undergoes left nasal septal biopsy
- Nasal septal biopsy: granulation tissue with scattered giant cells; no vasculitis seen
Nasal cultures: many S. aureus; oxacillin-susceptible - Laboratory results: positive ANCA screen, leading to further studies as below:
- ANCA titer: 1:40
- ANCA pattern: PANCA
- Myeloperoxidase antibody: 3 units (negative = 0-20 units)
- Proteinase 3 antibody: 47 units (moderate to strong > 30 units)
- Nasal septal biopsy: granulation tissue with scattered giant cells; no vasculitis seen
- 31 Aug 2010 - the culmination of her clinical signs, symptoms, biopsy and laboratory results, as well as a favorable response to prednisone suggests a diagnosis of Wegener's granulomatosis; patient is continued on prednisone and scheduled for frequent follow up in otolaryngology clinic. The severity of her disease is measured via her objective symptoms and by measurement of peak inspiratory flow (PIF). For more complete information regarding treatment, please see PIF chart below.
- 18 May 2011 - Patient undergoes first direct laryngoscopy with Kenalog injection, radial cuts, and balloon dilation of subglottic narrowing under general anesthesia after presenting to clinic with worsening shortness of breath and stridor, and a PIF of 1.76.
- 10 Aug 2011 - Patient undergoes second surgical dilation; PIF 1.51 prior to procedure.
- 25 Apr 2012 - Patient undergoes third surgical dilation; PIF 1.92 prior to procedure.
- 10 Oct 2012 - Patient undergoes fourth surgical dilation; PIF 1.59 prior to procedure.
- 13 Dec 2012 - Patient undergoes fifth surgical dilation; PIF 1.23 prior to procedure.
- 7 Feb 2013 - Patient is 11 weeks pregnant, and the decision is made to undergo tracheotomy for at least the duration of her pregnancy to avoid further surgeries; she undergoes awake tracheotomy the following day, 2/8/13 (PIF on 2/7/13 is 2.00).
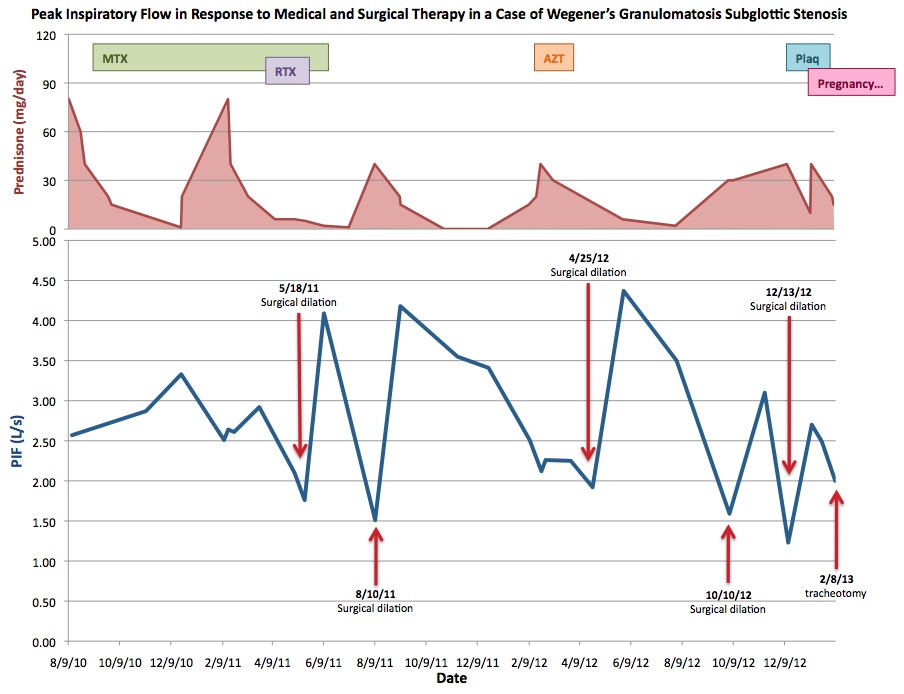
MTX: Methotrexate 10 mg weekly 9/10/10 - 12/24/10; 25 mg weekly 12/24/10 until discontinuation 6/16/11
RTX: Rituximab 375 mg weekly for four weeks starting 4/1/11 as part of RAVE protocol
AZT: Azathioprine 100 mg daily starting 2/23/11 until discontinuation 3/29/12 due to hepatic toxicity
Plaq: Plaquenil 300 mg daily starting 2/7/13 until discontinuation 4/11/13 due to concerns for pregnancy
Pregnancy: Conception in late November 2012



