Return to: Laryngeal leukoplakia white plaques on vocal cords
Go to: Mild squamous dysplasia causing laryngeal leukoplakia
Go to: Moderate squamous dysplasia causing laryngeal leukoplakia
Go to: Severe squamous dysplasia or Carcinoma in situ causing laryngeal leukoplakia
Go to: Invasive squamous cell carcinoma causing laryngeal leukoplakia
see also: Carcinoma-in-situ vs Severe Dysplasia
Squamous dysplasia is defined by the WHO as “altered epithelium with an increased likelihood for progression to squamous cell carcinoma (SCC).” It can show a variety of both architectural and cytological abnormalities (Table 1) that are considered in combination in order to assign a grade of mucosal disorder. The grade is related to the relative risk of developing an invasive carcinoma. At the University of Iowa, we use the 2005 WHO Classification scheme to categorize precursor lesions (Table 2).
Table 1. Architectural and cytological abnormalities present in dysplasia
|
Architectural changes |
Cytological changes |
|
Disordered cellular stratification |
Anisonucleosis (nuclear size variability) |
|
Loss of cellular polarity |
Nuclear pleomorphism (variation in nuclear shape) |
|
Mitotic figures above the basal zone |
Anisocytosis (cell size variability) |
|
Dyskeratosis (premature keratinization) |
Increased nuclear:cytoplasm (N/C) ratio |
|
Deep keratin pearl formation |
Atypical mitoses |
|
|
Nuclear chromatin alterations (hyperchromasia) |
Table 2. Classification schemes for grading squamous precursor lesions of the head and neck
|
2005 WHO Classification |
Squamous Intraepithelial Neoplasia (SIN) |
Ljubljana Classification Squamous Intraepithelial Lesions (SIL) |
|---|---|---|
|
Mild dysplasia |
SIN 1 |
Basal/parabasal cell hyperplasia |
|
Moderate dysplasia |
SIN 2 |
Atypical hyperplasia |
|
Severe dysplasia |
SIN 3 |
Atypical hyperplasia |
|
Carcinoma in situ |
SIN 3 |
Carcinoma in situ |
In general, we use the following rubric in assigning grade of dysplasia:
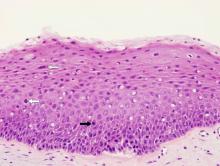
Mild dysplasia: Architectural disorder generally limited to the lower third of the mucosa with minimal cytological abnormalities. If mitoses are present, they are limited to the basal/parabasal layers. There is also no downgrowth of the mucosa. See example of mild dysplasia below.
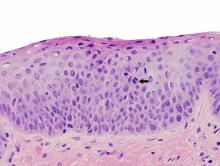
Moderate dysplasia: Architectural disorder extends into the middle third of the mucosa with accompanying more significant cytological abnormalities. Mitoses are generally limited to the lower portions of the mucosa. At this stage, the mucosa may show an abnormal epithelial-stromal interface and there may be downward growth of the mucosa. Whether this represents early invasion can be extremely difficult to determine. An important caveat to remember is that if the cytological changes are markedly abnormal, moderate dysplasia may be upgrade to severe dysplasia/carcinoma in situ. See example of moderate dysplasia below.
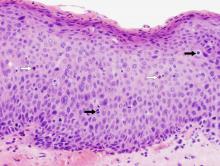
Severe dysplasia/Carcinoma in situ: While others attempt to separate these two grades of dysplasia, our preference is to consider them synonymous, as both have a significantly increased potential to be the final observable step before stromal invasion. Architectural disorder generally extends into the upper third of the mucosa and is accompanied by marked nuclear size variability, abnormal nuclear shapes, and nuclear hyperchromasia. Mitoses are generally in the upper layers of the mucosa and can even be atypical forms. At this stage, a more evident downward, bulging or budding growth pattern is observed indicating the presence of an altered epithelial-stromal interface. Careful attention to this junction is critical to exclude an early invasive carcinoma. See example of severe squamous dysplasia/carcinoma in situ below.
In considering all forms of dysplasia, we emphasize that a solid, collegial relationship be developed and maintained between the head and neck surgeon and surgical pathologist so that appropriate clinicopathologic correlation occurs whenever a leukoplakic lesion is biopsied and examined as the implications to the patient may be significant. If ever there appears to be a disconnect between the clinical impression and pathologic diagnosis, joint review, such as at a tumor board, is encouraged.
Examples of squamous dysplasias |
||
|---|---|---|
 |
 |
 |
|
Mild dysplasia: Architectural disorder generally limited to the lower third of the mucosa with mild nuclear pleomorphism and hyperchromatism. A mitosis (black arrow) and a couple dyskeratotic cells (white arrows) noted. |
Moderate dysplasia: Architectural changes extend well into the middle third of this biopsy with moderate nuclear pleomorphism, nuclear chromatin abormalities (coarse and hyperchromatic), and a middle layer mitosis (black arrow) are present. |
Severe dsplasia/carcinoma in situ: Near full thickness architectural disorder with marked nuclear pleomorphism and hyperchromatism, dyskeratosis (white arrows), and upper layer mitoses (black arrows). |
Conversion from glottis carcinoma in situ to invasive glottis cancer has been reported to occur in 5.4% of 3738 patients identified in the SEER database from 1988 to 2012 (Khaja 2016). When invasive cancer is subsequently found after treatment with a biopsy or excision of CIS it is difficult to distinguish between persistence from incomplete resection versus continued transformation of 'field-cancerized mucosa' (Gailey 2015). Other single institution retrospective cohort studies have described progression to invasive cancer in 11% (Sengupta 2010) and 9.6% (Karatayli-Ozgurosoy 2015). A pooled meta-analysis of 9 studies by Weller et al (2010) reported a 30% malignant transformation of severe dysplasia/CIS. The impact of continued smoking and alcohol consumption is thought to be an important variable increasing the risk of malignant transformation.
References
Khaja SF, Hoffman HT, and Pagedar NA: Treatment and Survival Trends in Glottic Carcinoma in Situ and Satge I Cancer From 1988 to 2012 Annals of Otology, Rhinology & Laryngology 125(4) 2016
Sengupta N, Morris CG, Kirwan J, Amdur RJ, Mendenhall WM. Definitive radiotherapy for carcinoma in situ of the true vocal cords. Am J clin Oncol. 2010;33(1):94-95
Weller MD, Nankivell PC, Mcconkey C, Paleri V, Mehanna HM. The risk and interval to malignancy of patiens with laryngeal dysplasia; a systematic review of case series and meta-analysis. Clin Otolaryngol. 2010;35(5):364-372
Karatayli-Ozgursoy s, Pacheco-Lopez P, Hillel AT, Best SR, Bishop JA, Akst LM. Larygneal dysplasia, demographics, and treatment: a single-instiution, 20-year review. JAMA Otolaryngol Head Neck Surg. 2015;141(4):313-318
“Leukoplakia” (Book Chapter) by Gailey M and Hoffman H in “Sataloff’s Textbook of Otolaryngology” edited by Dr. Robert Sataloff 2015