A multidisciplinary neuroscience study using rare, intraoperative brain recordings suggests that low frequency stimulation of a deep brain region may be able to improve cognitive function in patients with Parkinson’s disease (PD). The study findings, published Nov. 28 online in the journal Brain, also hint at the broader potential of brain stimulation for treating other cognitive diseases.
The new work by neurologists and neurosurgeons with the Iowa Neuroscience Institute at the University of Iowa provides the first direct evidence of a connection in the human brain between the thinking region of the brain (the frontal cortex) and a deeper structure called the subthalamic nucleus (STN) that is involved in controlling movement. The study also shows that stimulation of the STN at low frequencies improves the performance of PD patients on a simple cognitive task that is usually disrupted by PD.
“It’s not very often that you identify a new connection in the human brain,” says Nandakumar Narayanan, MD, PhD, UI assistant professor of neurology and senior study author. “The existence of this hyperdirect pathway from the prefrontal cortex to the STN has been bandied about for around a decade, but this is the first time we’ve experimentally shown that it exists and functions in people.
“We were also able to show that if we stimulate the STN, we change the frontal cortical activity and we think it’s by this pathway,” he adds. “And if we stimulate the STN and change cortical activity, we can actually change behavior in a beneficial way, improving the patients’ cognitive performance.”
Parkinson’s disease is a progressive neurodegenerative condition that affects about one million people in the United States. Deep brain stimulation of the STN at high frequencies is already approved to treat movement problems in some patients with PD. In addition to causing movement problems, however, PD also affects thinking. The new findings raise the possibility that STN deep brain stimulation at a different (low) frequency might also improve cognitive symptoms in PD, and possibly even in other neurologic and psychiatric diseases.
Nandakumar Narayanan, MD, PhD Jeremy Greenlee, MDListening in on the brain
The team was able to map the STN-cortex connection by “listening in” on brain activity during surgeries to implant deep brain stimulation (DBS) electrodes in patients with PD.
UI neurosurgeon Jeremy Greenlee, MD, conducts more than 30 such surgeries every year and his expertise was vital to the mapping experiment. Using specialized recording electrodes placed inside the patients’ brains, Greenlee listens in on brain activity in order to accurately place the DBS device. Those electrodes also allow direct recording of brain activity for experimental purposes in patients who are awake during the procedure without adding any risk. This kind of intraoperative recording is not very common, but Greenlee and his UI colleagues have a long history of expertise in the technique.
During the surgery, the patients did a simple cognitive task as a way of stimulating one part of the brain while recording electrical activity from other parts that are connected. Listening to the neural activity during the task allowed the team to map the connection.
“We were able to evoke a response to show the functional connection,” Greenlee explains. “The very fast response suggests a single, direct synaptic connection—that is what hyperdirect means.”
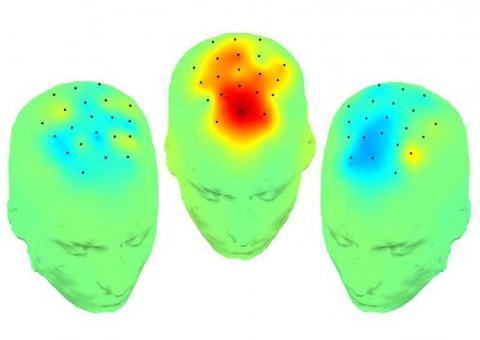
The image illustrates changes in prefrontal brain activity when the subthalamic nucleus (STN) is stimulated at: high frequencies (right head), not stimulated at all (left head), or stimulated at 4 Hz (middle head). Stimulating the STN at 4 Hz produces a significant increase in frontal activity (also at 4 Hz), which correlates with improved patient performance on a simple cognitive task that is usually disrupted by PD. The findings by neuroscientists at the University of Iowa and published Nov. 28 in Brain, suggest that low frequency stimulation of the STN may provide an approach for treating cognitive symptoms of PD, and possibly other cognitive diseases.
Stimulation improves cognitive performance
Having established the existence of the hyperdirect connection, the researchers next investigated the effect of low frequency STN stimulation on cognitive abilities. Narayanan’s team uses a very simple thinking task—accurately estimating the passage of a short interval of time—to study cognitive impairment in PD patients and animal models of PD.
During post-surgery follow up visits, the researchers had the patients do the interval timing task with the DBS stimulator set to one of three settings: high frequency (normal for controlling movement), no stimulation, or a low frequency setting of 4 Hz. Only the 4 Hz stimulation improved the patients’ performance on the timing test.
Previous research from Narayanan’s labs has shown that people with PD and rodent models of the disease are missing a specific brain wave known as the delta wave in their frontal cortex while they are doing the timing task. The delta wave cycles at a frequency of about 4 Hz.
“When we stimulate the STN at 4 Hz, the delta wave is restored in the mid frontal cortex,” Narayanan says. “By stimulating the STN we can rescue cortical activity (which is disrupted in PD), and we can improve cognitive behavior.”
The researchers think the frequencies are like communication channels between networks. If two networks are working together at the same frequency, that might be a unique way the networks interact and information is transmitted.
“The fact that we are able to test a lot of our ideas (that come from the rodent studies) about how the neural networks work in awake behaving humans, is something I never dreamed I’d be able to do, but it enables us to ask questions that might actually help a lot of people,” Narayanan says.
“It is exciting to potentially have a way to improve cognition that could be life changing for patients,” Greenlee adds.
In addition to Narayanan and Greenlee, the UI study team included Ryan Kelley, Oliver Flouty, Eric Emmons, Youngcho Kim, Johnathan Kingyon, Jan Wessel, and Hiroyuki Oya.
The study was funded in part by a grant from the National Institute of Neurological Disorders and Stroke (NINDS) to Narayanan (R01 NS100849). Greenlee receives funding from the National Institute on Deafness and Other Communication Disorders. (NIDCD).