Using gene therapy to treat cystic fibrosis
A few years after the cystic fibrosis (CF) gene was discovered, University of Iowa scientists were the first to report that gene therapy could partially correct the CF ion channel defect in people.
The UI experiments involved nasal delivery of a modified adenovirus vector carrying the corrected cystic fibrosis transmembrane conductance regulator (CFTR) gene. Although initial results were promising, it was soon apparent that with each dose, the patient gradually developed immunity to the virus, making it ineffective for long-term therapy.
Still, gene therapy research quickened across the country until 1999, when the tragic death of a participant in a gene therapy clinical trial for a disease known as ornithine transcarbamylase deficiency essentially shut down human trials and sent scientists back to the drawing board.
Problems with gene therapy
In theory, CF is a perfect target for gene therapy. The condition is caused by well-studied mutations in a single gene. Even partial restoration of normal CFTR protein function appears to produce therapeutic benefit. In addition, lung cells affected by CF line the airway surface and are potentially accessible to an inhaled gene therapy.
Two of the biggest barriers are how to achieve safe, efficient, and sustainable gene expression and how to precisely deliver gene therapy to the right cells. Led by Joseph Zabner, MD, Paul McCray, MD, and John Engelhardt, PhD, the UI group has refined its understanding of these barriers and developed solutions.
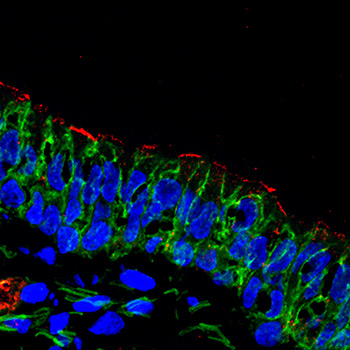 Finding a solution
Finding a solution
In the image: After gene therapy delivery, CFTR protein (red) is present at the surface of airway cells. This CFTR replacement improves the ability of airway secretions to kill bacteria.
“Viruses, like lentivirus, parvoviruses, and adenoviruses, already have millions of years of evolution behind them and know how to get into cells and deliver their genetic cargo. We’re just using our understanding to redesign them to deliver therapeutic cargoes,” McCray says.
With pig and ferret models of CF, researchers now have animal models for testing both the efficiency of gene therapy and the potential therapeutic effects of restoring gene expression.
Most recently, the Iowa researchers showed that two different viral vectors can restore a working version of CFTR to pigs’ airway cells. Importantly, the gene replacement normalizes key aspects of the lung biology and improves the ability of airway secretions to kill bacteria.
The future of gene therapy for CF
“Gene therapy development has experienced ups and downs, and for CF there has been a long period with few clinical studies,” McCray notes. “But a nonviral gene therapy trial was completed recently in the U.K., and we’ve also started to see successes in other single-gene diseases. With these results and all we have learned about getting the CFTR gene into the right place, there is renewed interest in moving forward with CF gene therapy again.”
In 2017, the Food and Drug Administration approved gene therapy treatments for certain blood cancers and for a type of inherited blindness. Given these advances, and the potential opportunity for using optimized viral vectors to deliver gene-editing cargo as well as corrective genes, there is a sense among the UI team that gene therapy may finally be at a tipping point.
“There has been tremendous progress in the delivery technology,” Zabner says. “I think we’re at a point where we’re going to start seeing a new round of therapeutic trials with the next generation of delivery tools.”
By Jennifer Brown
View other features on cystic fibrosis research
Featured in Medicine Iowa Spring 2018
You're reading one of the features included in the Spring 2018 issue of Medicine Iowa. Read more news and features about the people and programs focused on teaching, healing, and research in the UI Roy J. and Lucille A. Carver College of Medicine.