Iowa Thoracic Protocols
Evgeny V. Arshava, MD, John C. Keech, MD, Melissa Tvedte, ARNP, Kelley Mclaughlin, RN, Joan Ricks-McGillin, RN, Kalpaj R. Parekh, MBBS
Please direct questions and comments to Evgeny V. Arshava, MD evgeny-arshava@uiowa.edu
See also other sections in Thoracic Surgery
Cervical gastroesophageal anastomosis (modified Collard) operative technique
Reconstuction after total laryngectomy (with pharyngo-esophageal anastomosis)
Esophagoscopy with narrow band imaging (NBI) for Reflux Esophagitis
Esophageal Myotomy and Diverticulectomy for Epiphrenic and Midesophageal Diverticula
For general considerations, basic anatomy and choice of the approach for an esophagectomy see: Esophagectomy for an Esophageal Cancer: General Considerations and Choice of an Operation
INDICATIONS
-
Carcinoma of the upper, middle, and lower third of the esophagus
-
Early-stage (T1a) carcinoma of the mid and distal esophagus not amenable to endoscopic mucosal resection and low risk T1b- T2, NO
-
Carcinoma of the mid and distal esophagus T1b–T4a after preoperative chemoradiation
-
End-stage benign strictures of the esophagus not amenable to transoral dilations (caustic injury, peptic structures)
CONTRAINDICATIONS
-
Tracheobronchial, mediastinal, and intraabdominal structures invasion (T4b)
-
Stage IV disease (with exception of selected patients with limited celiac lymph node metastasis—M1a stage IVa)
-
Advanced physiologic (not chronologic) age and frailty
-
Prohibitive co-morbidities
ANESTHESIA CONSIDERATIONS
General
-
Double-lumen endotracheal tube
-
Two large-bore peripheral intravenous accesses
-
Radial arterial line
-
Central venous line for selected patients only (via right neck)
-
Type and cross
-
Cefazolin within 1 h of the incision
Specific
-
Patient positioned in the left decubitus position on the bean bag
-
After completion of the thoracic portion of the operation, patient is repositioned supine with the shoulder roll, neck slightly hyperextended and turned right
-
Arms tucked
-
Single-lumen endotracheal tube
-
Mean arterial pressure (MAP) is kept >65 mmHg (to optimize conduit perfusion)
-
No vasopressors can be started without notifying the surgeon. Hypotension can almost often be managed with volume expansion.
NURSING CONSIDERATIONS
Instrumentation and Equipment
Standard
-
Major chest tray
-
Thoracic tray
-
Thoracoscopy equipment and tray
-
Energy device
-
The table-mounted retractor
-
Endoscopic linear cutting stapler
Special
-
Lighted transhiatal retractor (narrow and wide in standard and bariatric depths)
-
Saratoga sump drain (for gastric conduit positioning)
-
Doppler probe (available in room for obese patients)
-
Magnetic nasal bridle
Medications
-
Botox 200 units diluted in 8 mL of normal saline (optional per surgeon preference)
Prep and Drape
-
Standard prep using chlorhexidine solution
-
Field includes:
-
Thoracic phase—supraclavicular area to below costal margin, spine to nipple line
-
Abdominal and neck phase—mandible to pubis, between bilateral midaxillary lines, including both shoulders
-
Neck is draped just below the left ear lobe
-
Transparent incise drape (optional)
-
Drains and Dressings
-
Jackson-Pratt bulb drains, or Penrose drains
-
32 Fr chest tubes × 2
-
16 or 18 Fr nasogastric (NG) tube
OPERATIVE PROCEDURE
Thoracic phase (options of thoracotomy or thoracoscopy)
-
requires single lung ventilation via double-lumen endotracheal tube
Thoracoscopy
-
Thoracoscopic ports are placed typically:
-
Camera port—6th or 7th intercostal space on posterior axillary line
-
Working port #1—4th or 5th intercostal space on the middle or posterior axillary line
-
Working port #2—8th or 9th intercostal space on just anterior to the scapular line
-
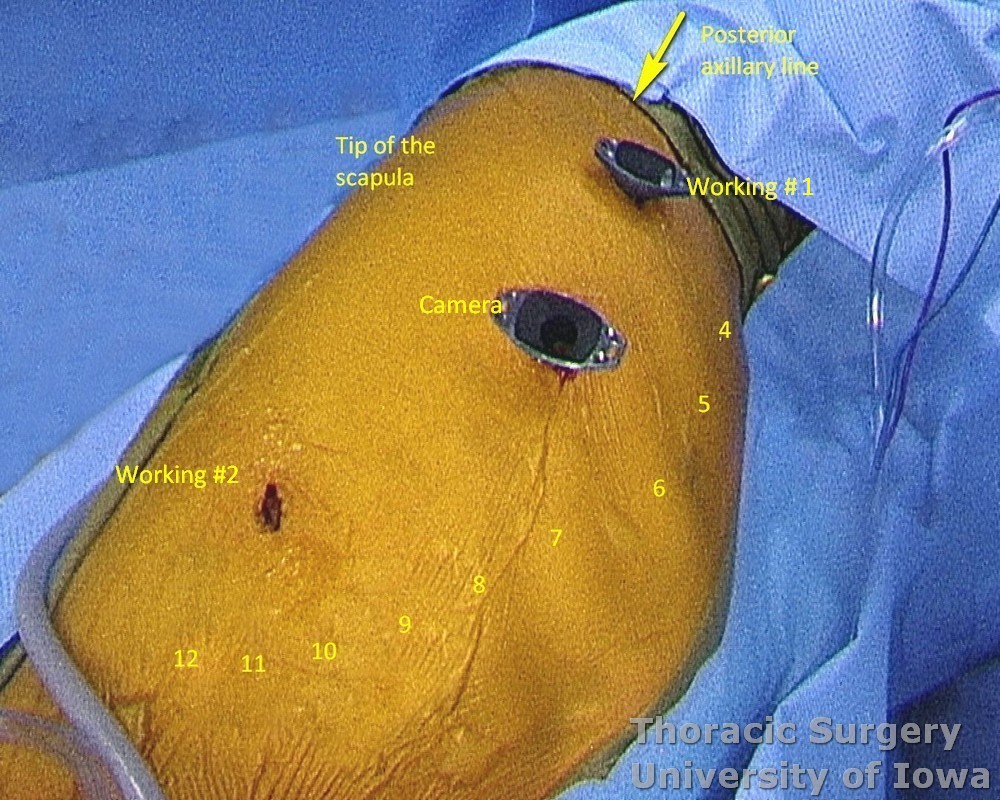
-
Note that as the area of dissection changes, the camera is repositioned between port sites as needed
-
Thoracoscopy is performed to exclude metastatic disease and identify relevant anatomic structures.
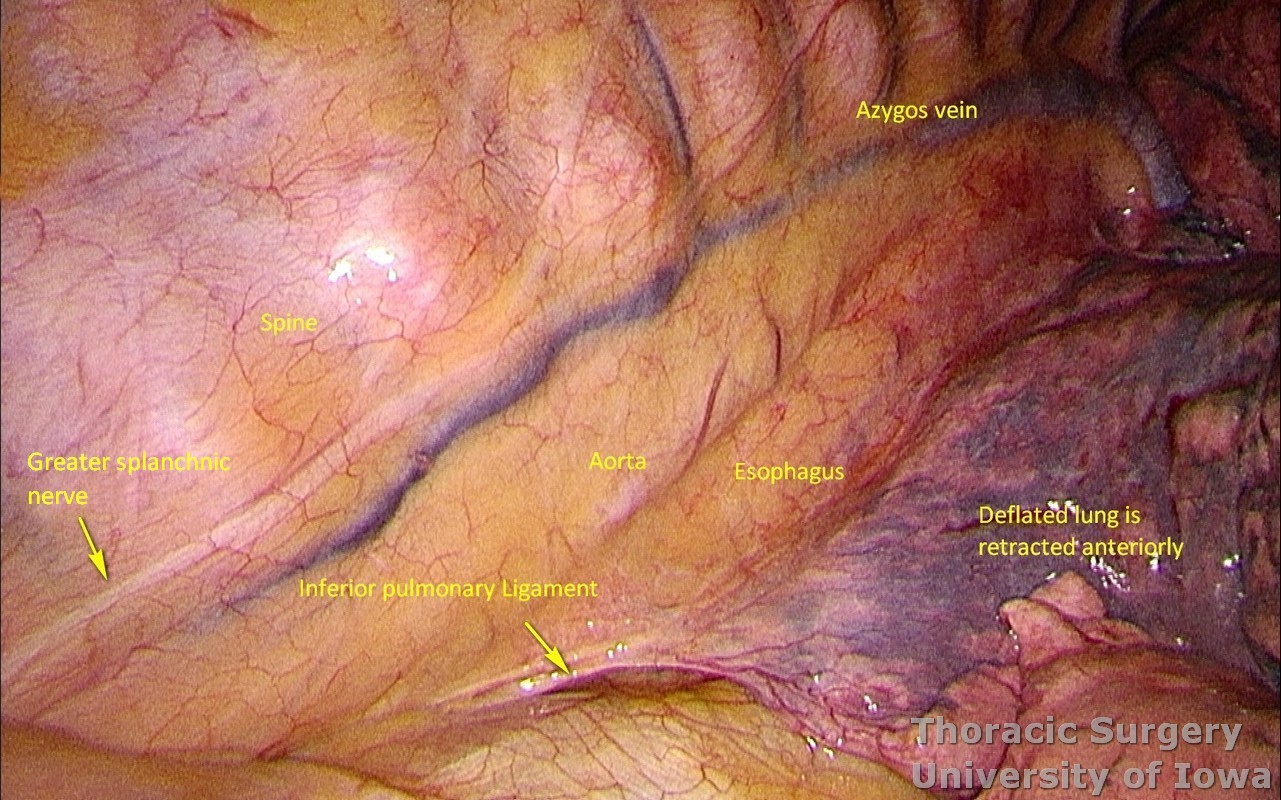
-
The deflated lung is retracted anteriorly for exposure of the posterior mediastinum.
-
The diaphragm is retracted as needed with a ring forceps or with a retraction stitch through port site #2.
-
The inferior pulmonary ligament is divided.
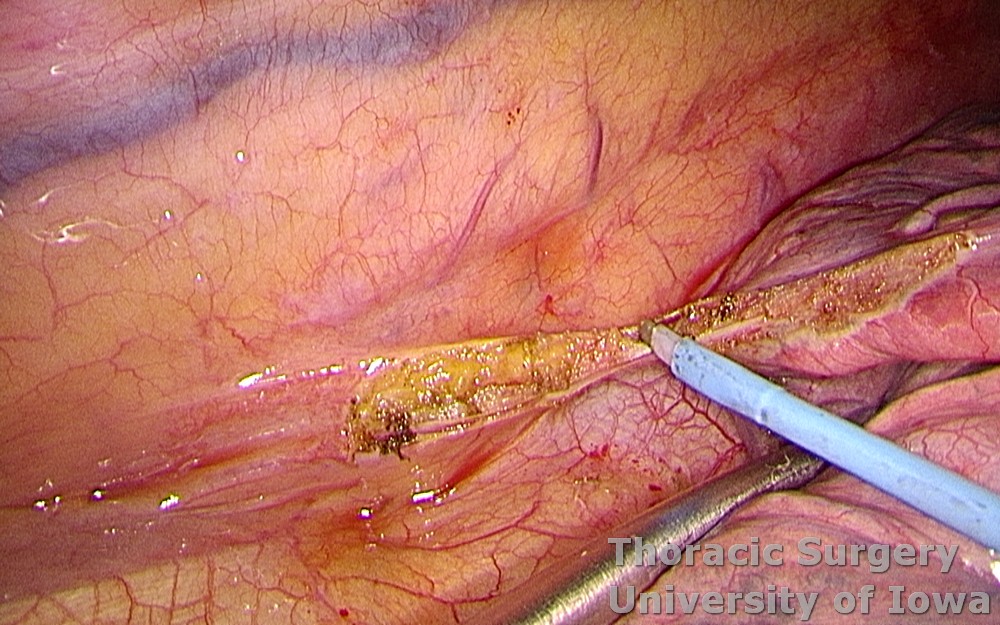
-
The parietal pleura is incised along the esophagus medially along the pericardium, and laterally below the aorta.
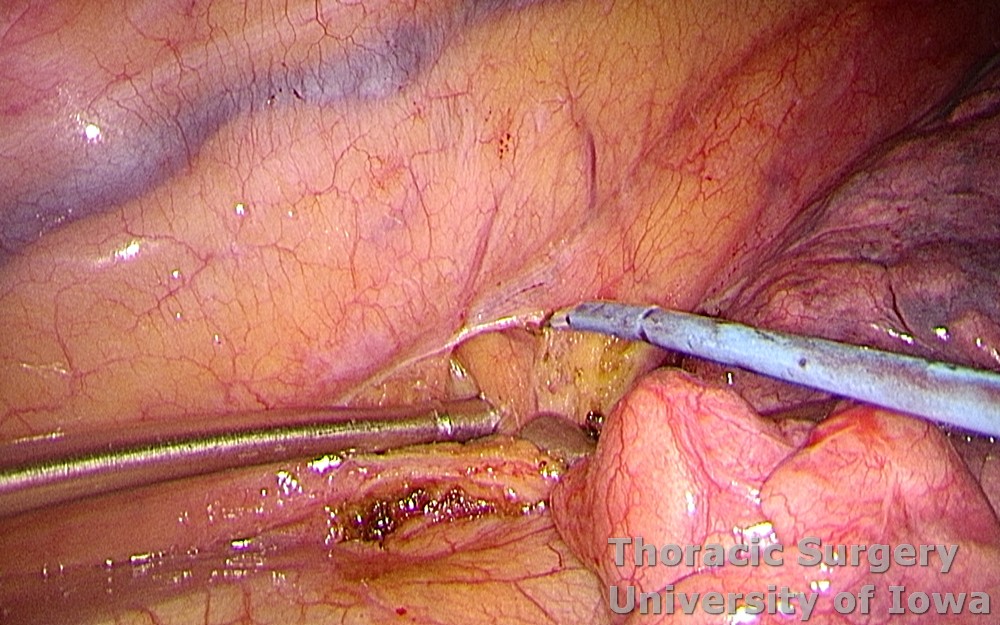
-
During latera dissection, the aorta and thoracic duct (See Thoracic duct and chylothorax) are protected.
-
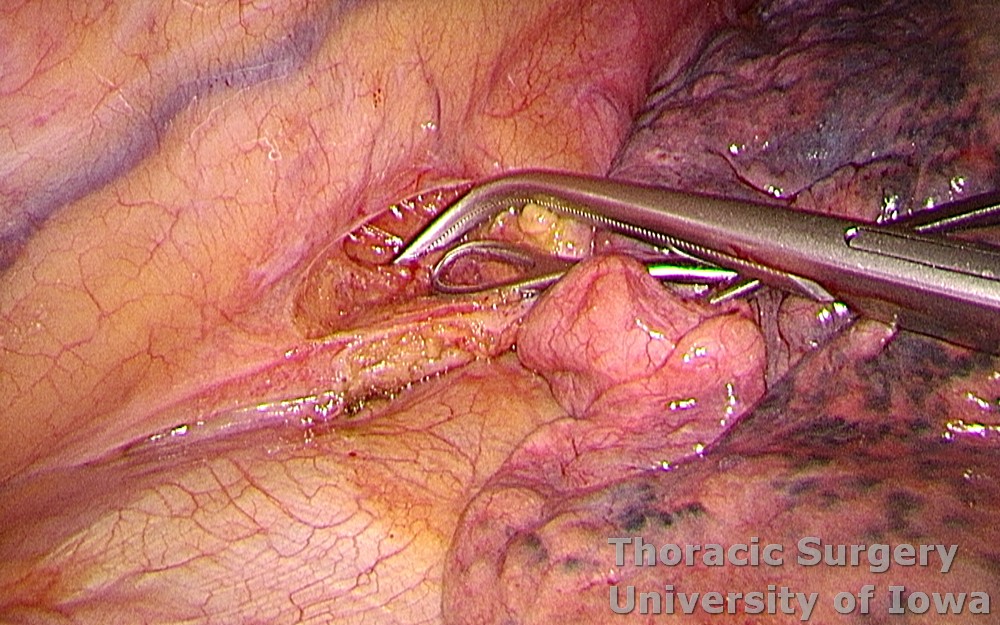
The esophagus is dissected circumferentially and encircled with the Penrose drain to facilitate retraction.
-
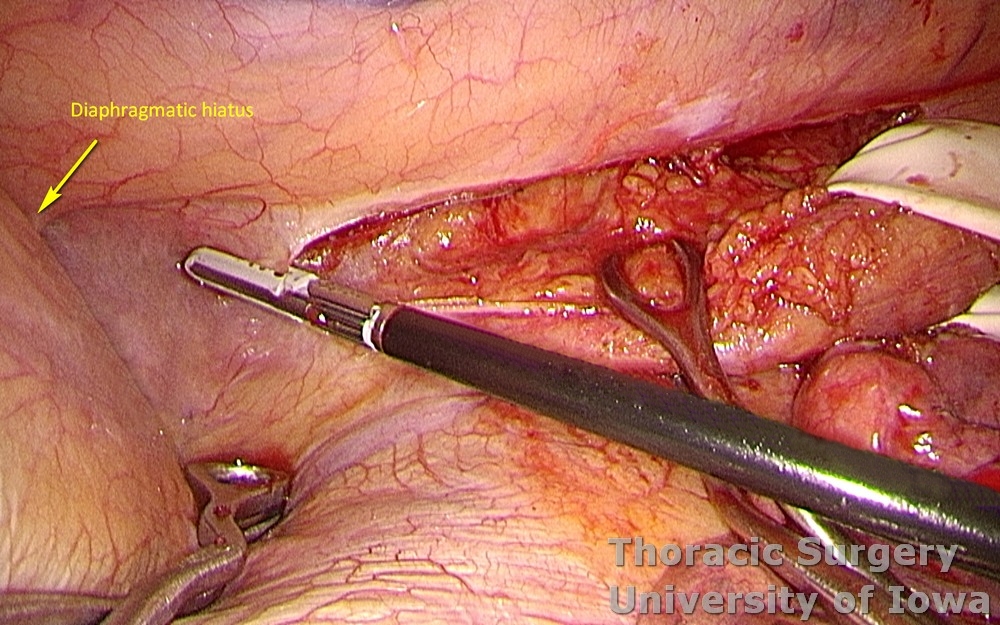
The distal esophagus is dissected towards the hiatus.
-
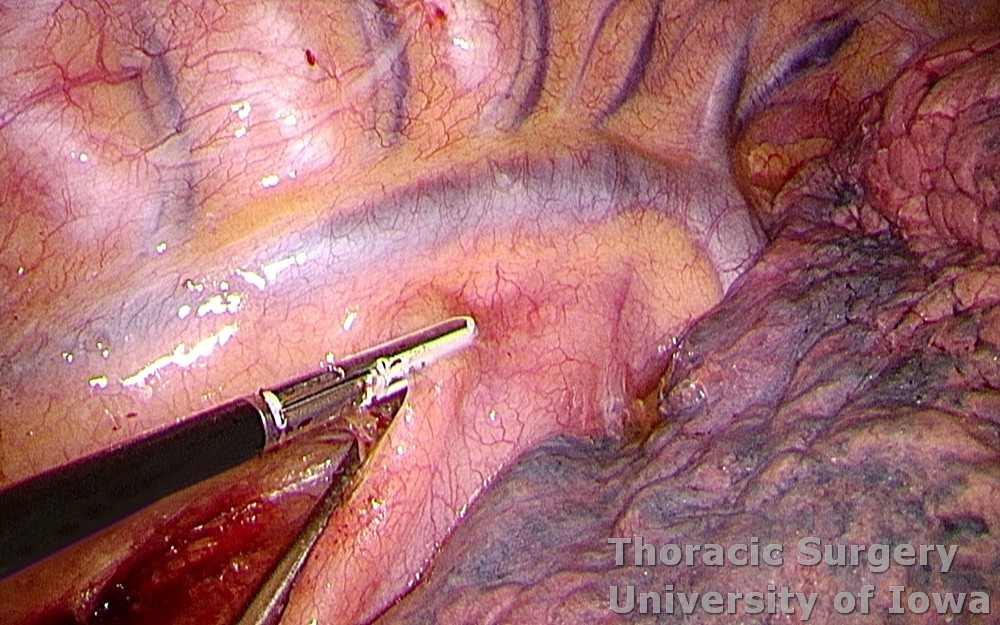
Dissection is carried cephalad.
-
Inferior pulmonary vein and the bronchus intermedius are identified and protected medially.
-
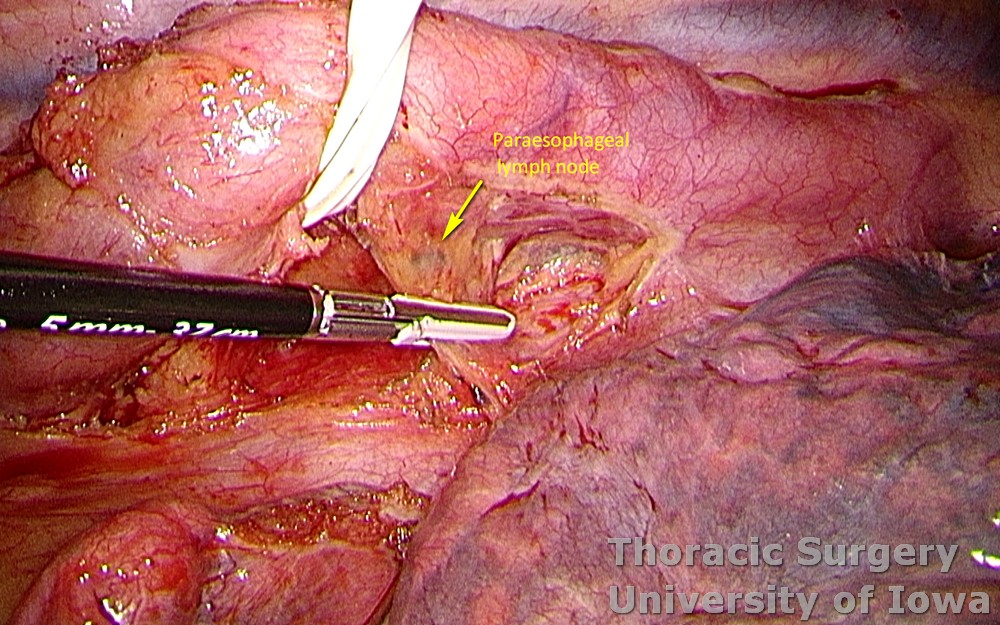
The paraesophageal and subcarinal lymph nodes packets are incorporated with the specimen or dissected separately.
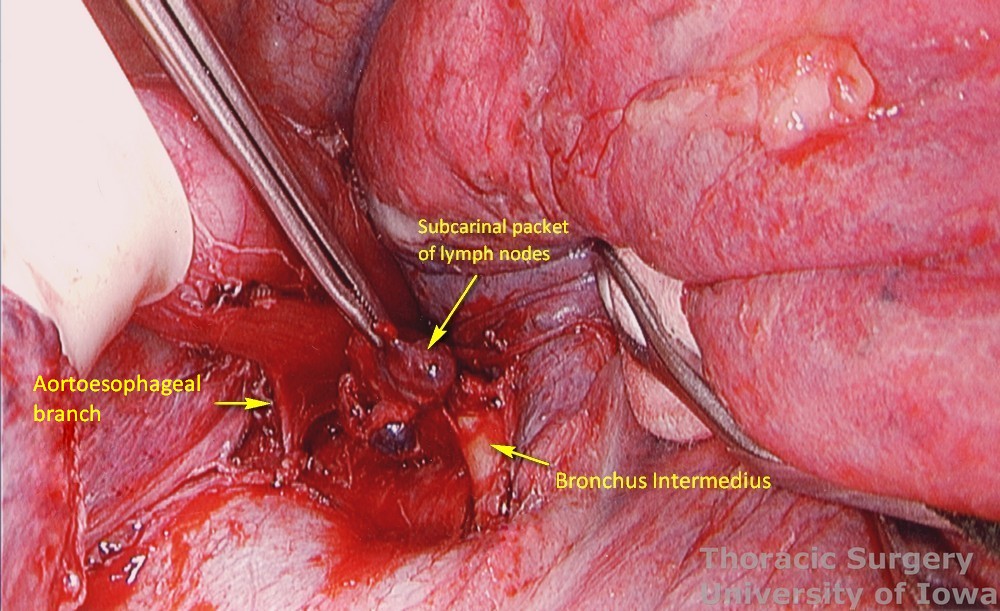
-
Energy device use is minimized in the area of the membranous airway.
-
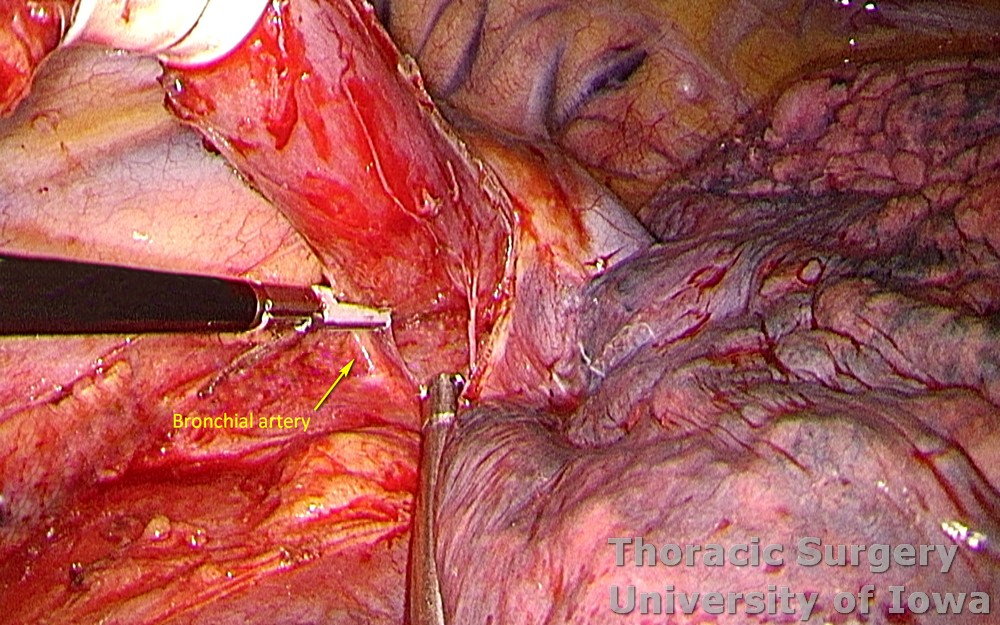
Aortoesophageal branches and one or two prominent bronchial arteries need to be controlled in the subcarinal area.
-
Clips are used liberally to control aortoesophageal branches and larger lymphatics.
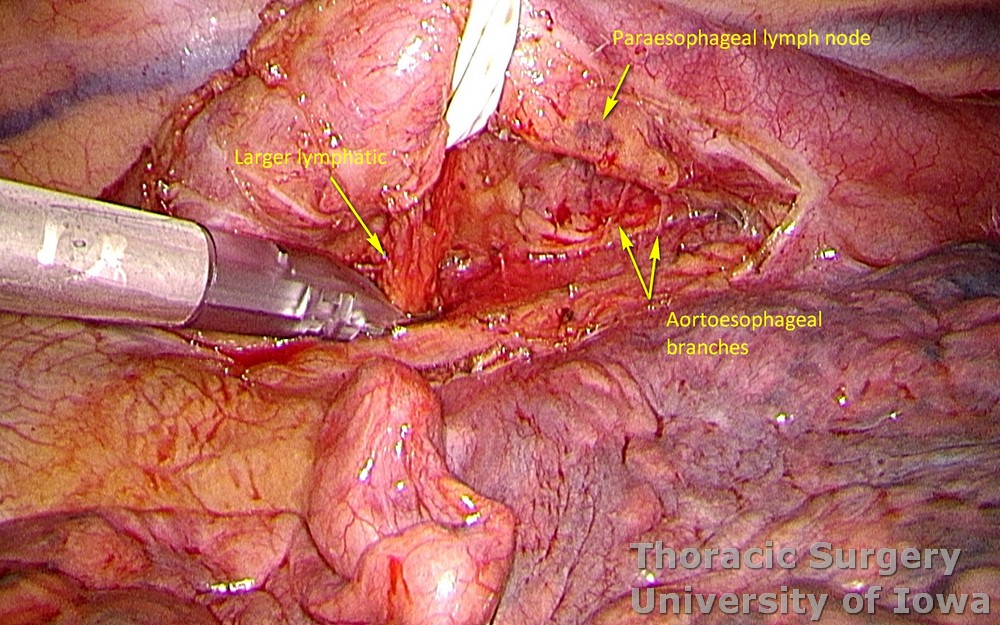
-
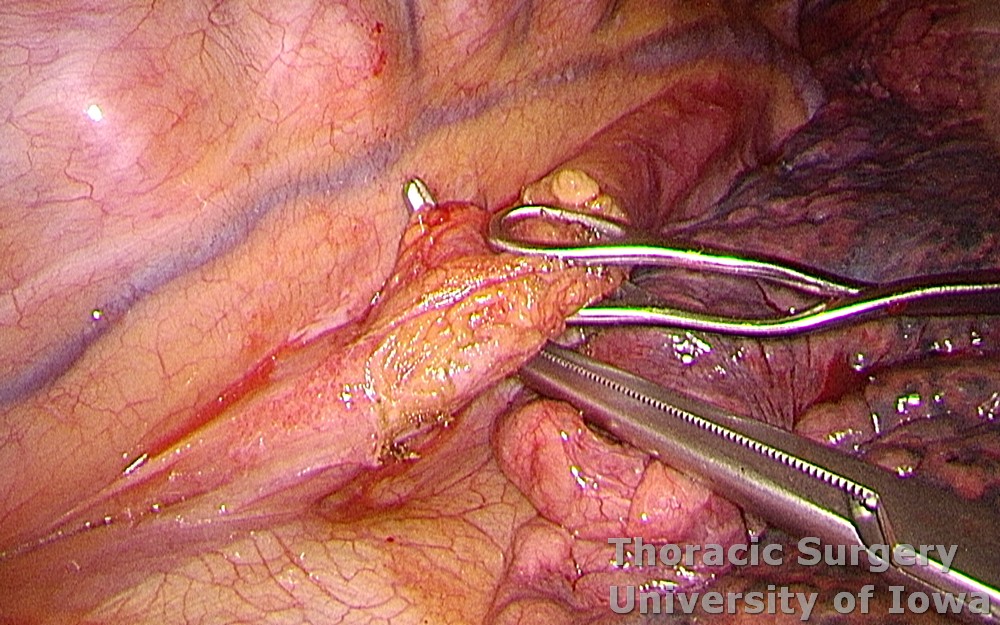
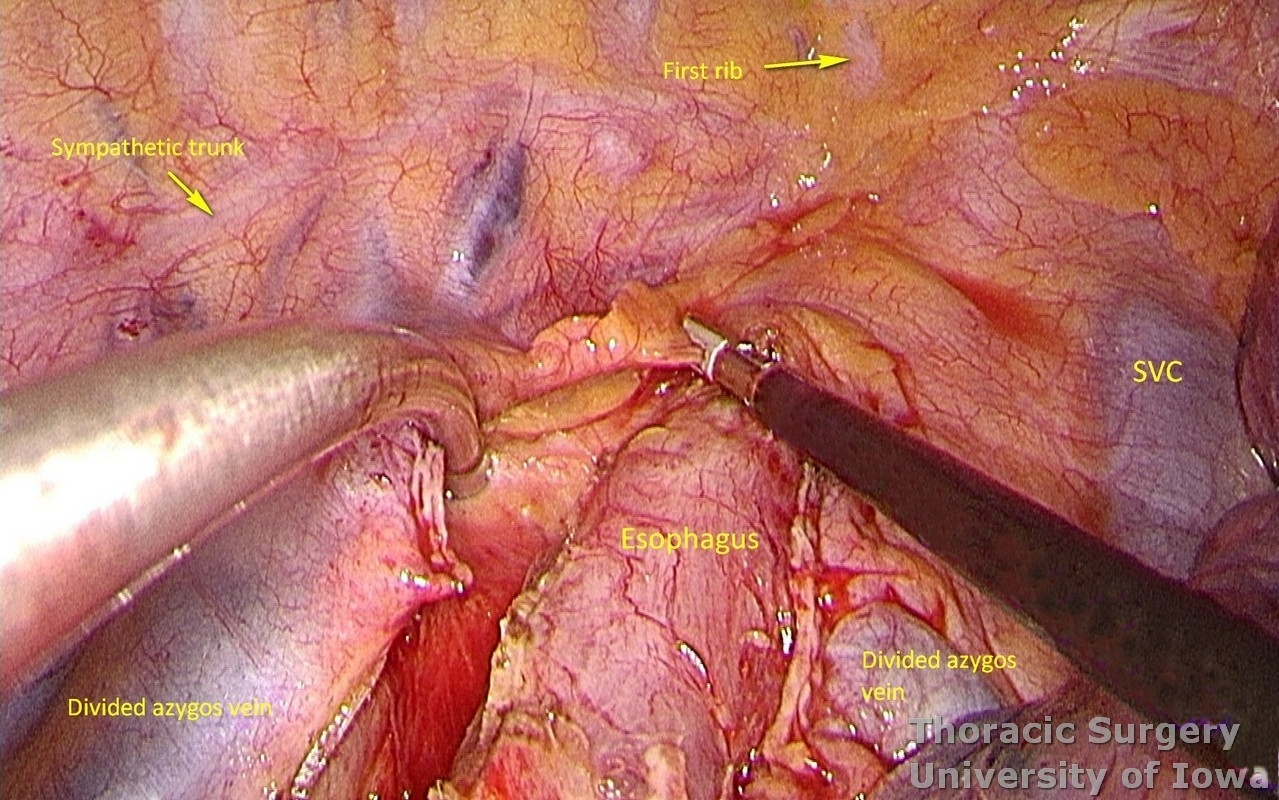
The azygous vein is dissected and divided with a linear cutting stapler.
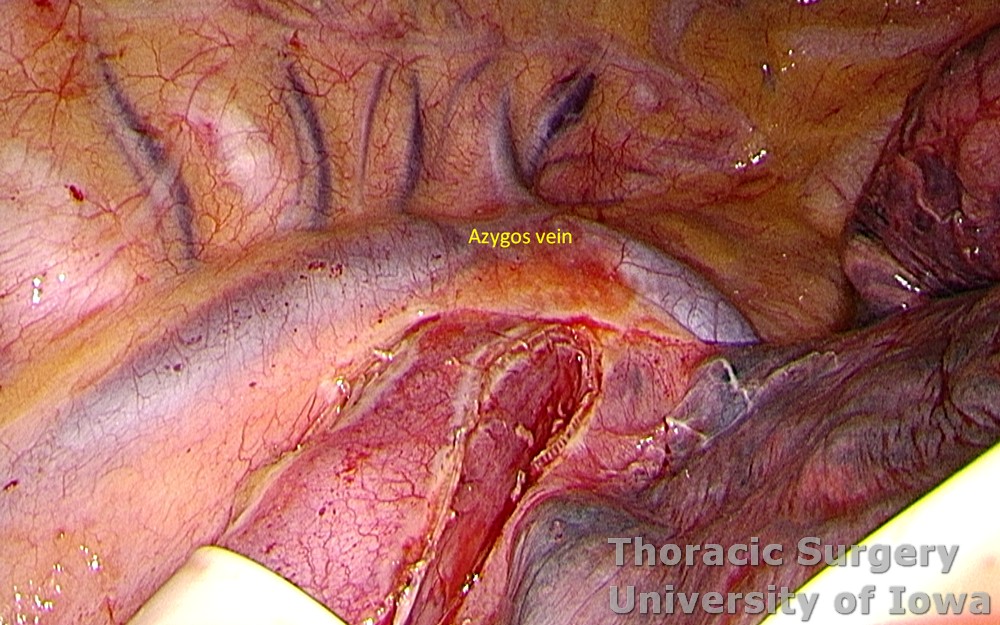
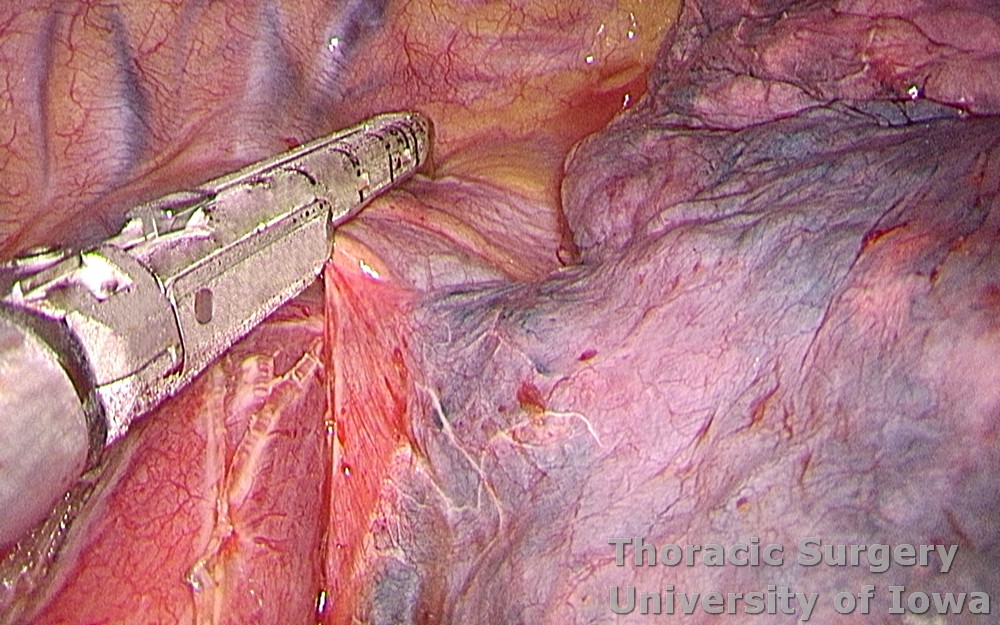
-
The right vagus nerve is divided just above the azygos vein and reflected anteriorly to avoid traction of the recurrent laryngeal nerve (RLN) superiorly.
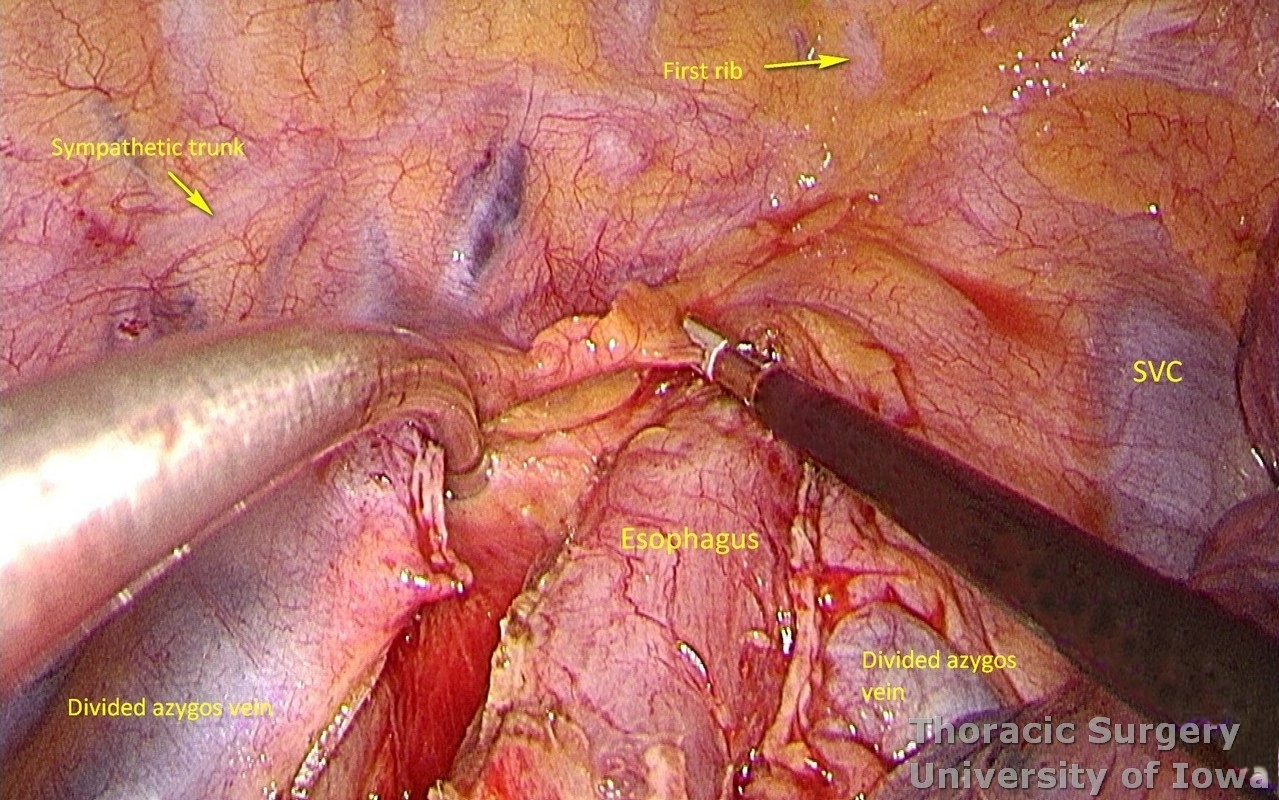
-
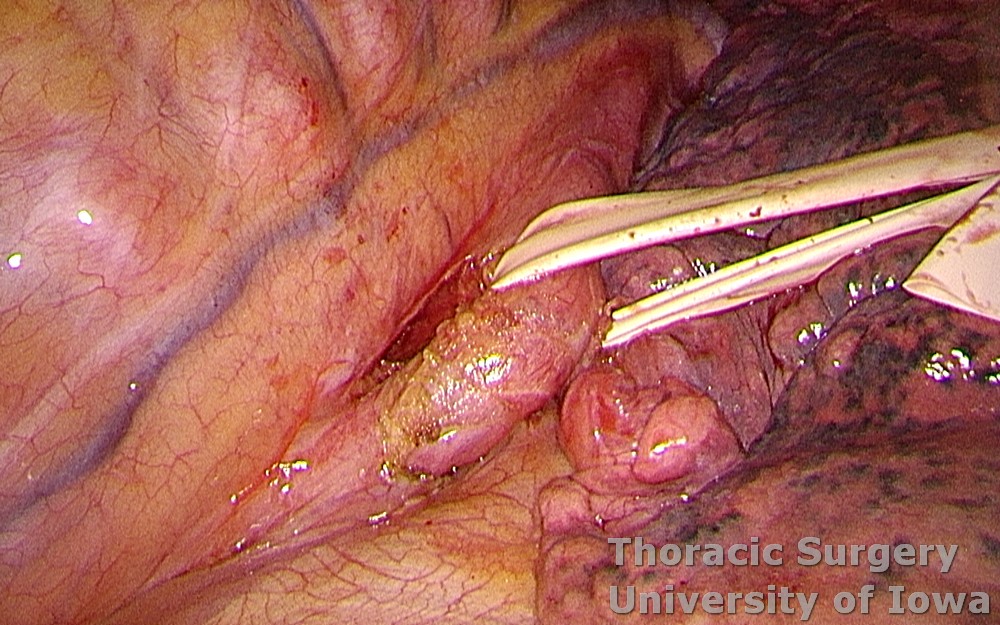
Dissection is continued towards the thoracic inlet, where small upper paraesophageal lymph nodes may be encountered.
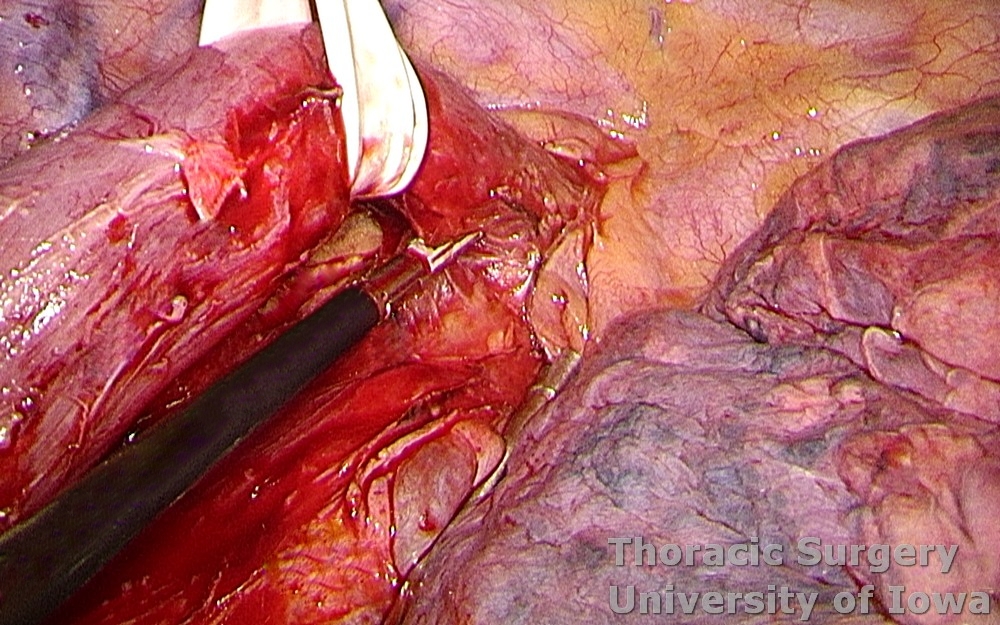
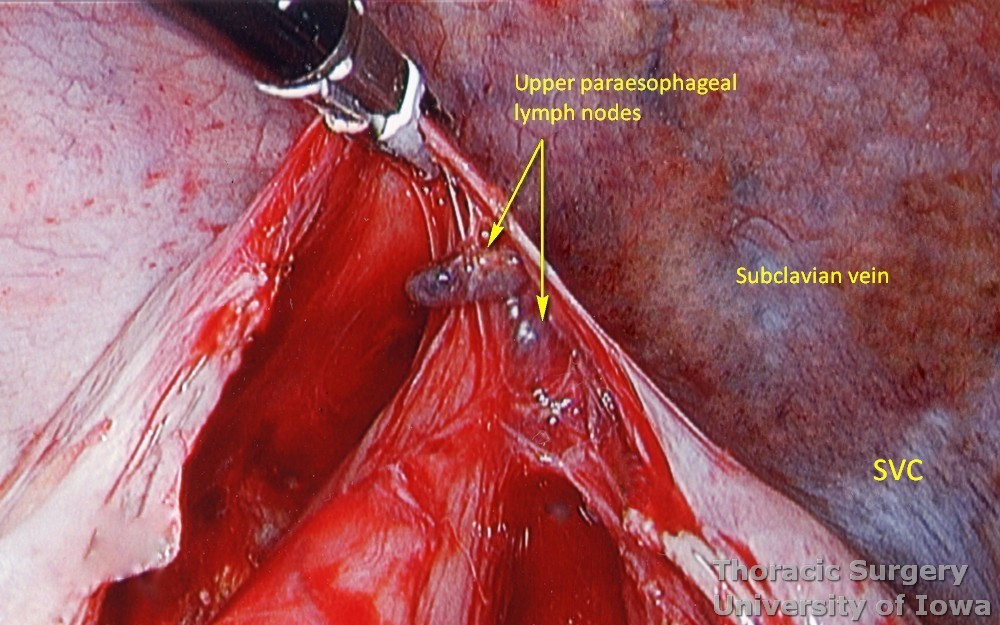
-
The thoracic esophagus is completely mobilized.
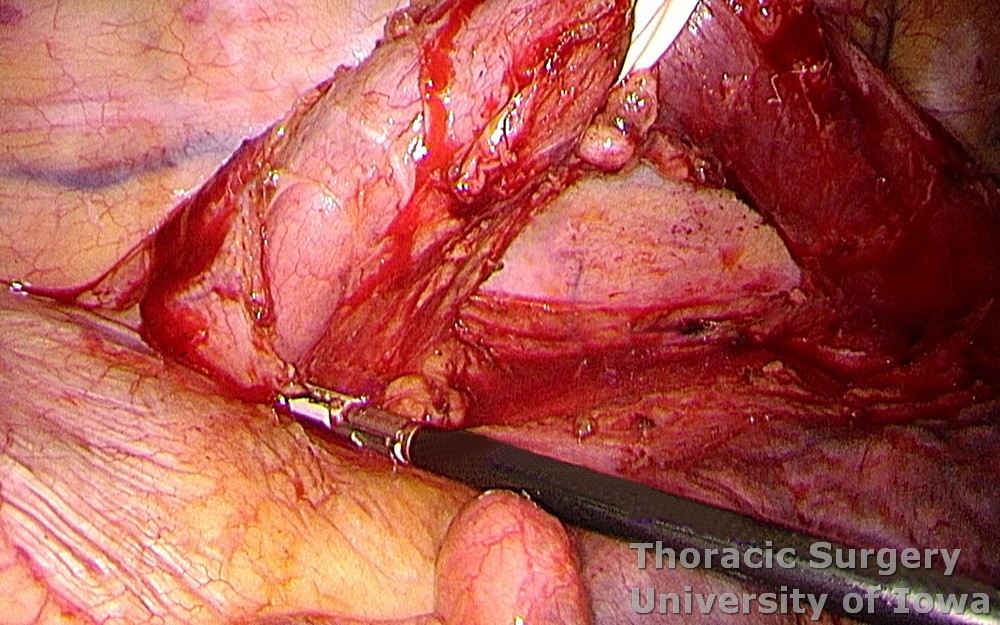
-
The chest tube is placed and the port sites are closed with absorbable suture.
Thoracotomy
-
Thoracotomy is reserved for bulky midesophageal tumors, cases of esophageal perforations, redo chest surgery or history of prior radiation.
-
A right posterolateral thoracotomy is performed through the 5th or 6th intercostal space with division of latissimus dorsi muscle and preservation of serratus anterior muscle (see Posterolateral thoracotomy techniques).
-
The deflated lung is retracted anteriorly for exposure of the posterior mediastinum.
-
The parietal pleura is incised and the inferior pulmonary ligament is divided.
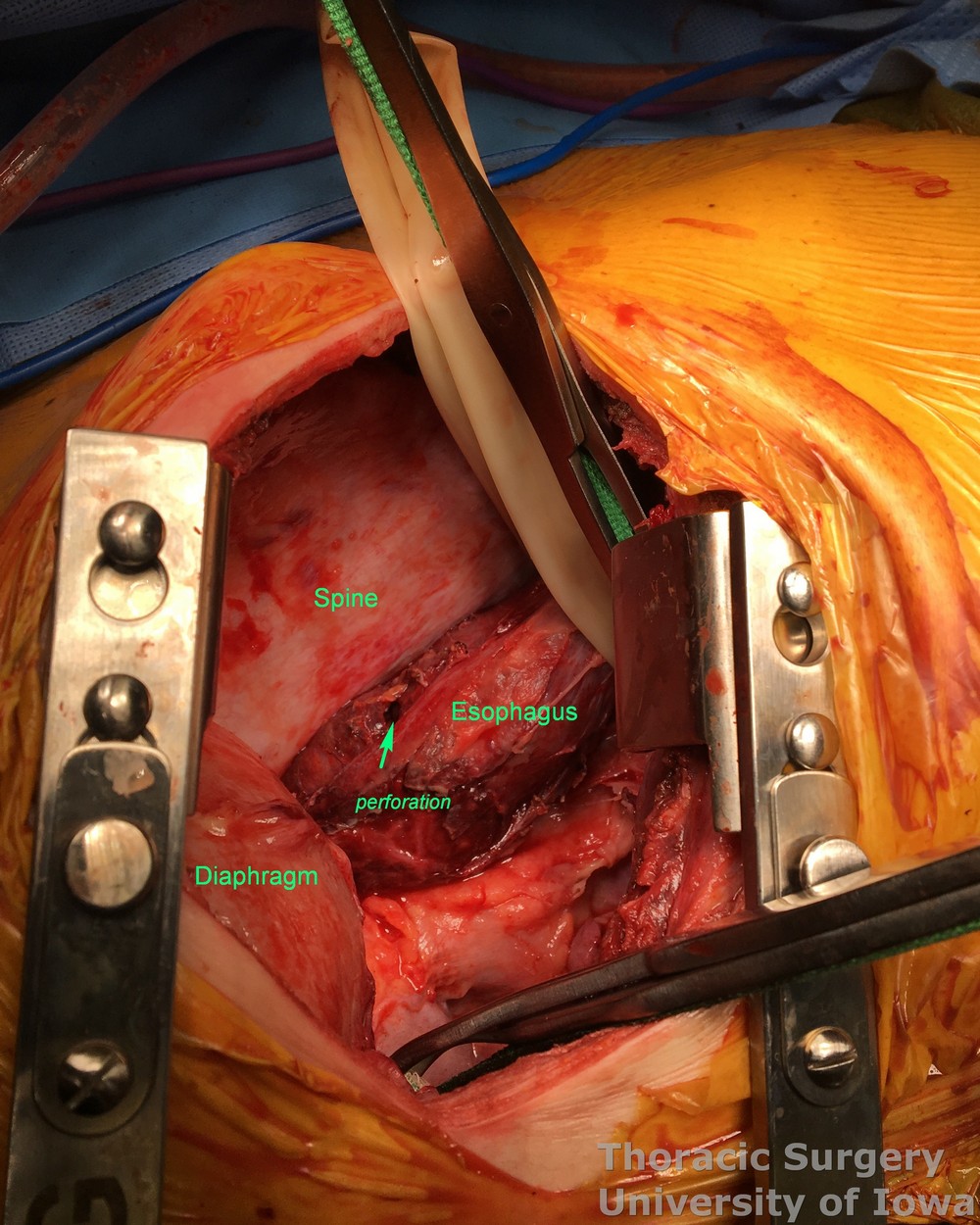
-
The azygous vein is divided with a linear cutting stapler or sharply between ties.
-
The esophagus is dissected circumferentially from the level of the hiatus into the thoracic inlet. Paraesophageal and subcarinal lymph nodes are incorporated with the specimen or dissected separately.
-
The esophagus is further dissected bluntly with a finger into the thoracic inlet.
-
A chest tube is placed and the thoracotomy is closed in layers with absorbable suture.
Abdominal phase (for abdominal phase operative images, see Transhiatal esophagectomy page)
-
A supraumbilical incision is made. An optional excision of the xiphoid process for deep and narrow chest may be considered.
-
Division of the falciform and left triangular ligament is made to retract the left lobe of the liver.
-
The table-mounted retractor is deployed and abdominal exploration for metastatic disease is performed.
-
The pars flaccida is incised and the gastrohepatic ligament is completely opened towards the right crus. The right gastric artery is preserved if possible.
-
The replaced/accessory left hepatic artery if present can be divided.
-
The phrenoesophageal ligament is divided and the right crus dissected.
-
The abdominal esophagus, periesophageal fat, and nodes are dissected and encircled with a Penrose drain for retraction.
-
The hiatus is opened and circumferential mediastinal periesophageal dissection is performed, reaching the area of dissection completed through the chest.
-
For distal esophageal tumors, manual palpation through the hiatus is performed to assess mobility of the esophagus. The tumor is “rocked” from side to side to make sure it is not adherent to the aorta, prevertebral fascia, or mediastinal structures. Is cases with suspicious preoperative imaging, the abdominal exploration may thus be needed to be performed prior to the thoracic phase.
-
The course of the right gastroepiploic artery is determined. The gastrocolic ligament is incised in its avascular portion between the terminal branches of the right and left gastroepiploic vessels and the lesser sac is entered. The gastrosplenic ligament is divided towards hiatus, dividing the short gastric vessels with an energy device.
-
The greater curvature of the stomach is then mobilized towards the pylorus, dividing the gastrocolic ligament no closer to the vessels than 1.5-2 cm, while protect gastroepiploic vessels with fingers of the retracting hand.
-
Adhesions of the stomach to the retroperitoneum and posterior gastric artery, if present, are taken down to protect pancreas.
-
In morbidly obese patients right gastroepiploic artery may not be visible or palpable. Doppler probe is used then to identify its course and origin from the gastroduodenal artery.
-
Once the stomach is fully mobilized and reflected anteriorly, the left gastric vascular pedicle is identified and divided with an endoscopic linear cutting stapler proximally, including all adjacent lymph nodes in the specimen.
-
Adequate mobility of the stomach is assured by the ability to bring the pylorus towards the hiatus.
-
Kocher maneuver is almost never needed.
-
Injection of 200 units of Botox diluted in 8 mL of normal saline is performed anteriorly and posteriorly into pylorus (divided in 2 mL portions in four quadrants) to improve gastric emptying postoperatively. No pyloromyotomy is needed.
-
Alternatively, pyloromyotomy can be performed using an electric cautery to divide the pyloric muscle.
Cervical phase (for cervical phase and anastomosis operative images see Cervical gastroesophageal anastomosis page)
-
A 6 cm skin incision is made along the anterior border of the left sternocleidomastoid muscle, starting at the sternal notch and extending to the level of the cricoid cartilage.
-
The platysma is divided and dissection is continued medially to the sternocleidomastoid muscle, carotid sheath, and laterally to the trachea and the thyroid.
-
The omohyoid muscle is divided with electrocautery.
-
The strap muscles are divided with electrocautery.
-
The middle thyroid vein and the inferior thyroid artery are divided.
-
Care is taken to protect the recurrent laryngeal nerve. Use finger to retract the trachea and not the metal retractor.
-
The deep cervical fascia is incised and, with further dissection towards the vertebral bodies, the esophagus identified, gently mobilized circumferentially, and encircled with a Penrose drain.
-
The esophagus is further dissected into the superior mediastinum with gentle traction and finger dissection, reaching the dissection completed during the chest phase.
-
The esophagus is divided with a linear stapler in the neck incision (the NG tube is pulled back), preserving as much cervical esophagus as possible.
Conduit preparation and positioning
-
The stomach and thoracic esophagus are delivered out of the abdominal incision.
-
Lighted transhiatal retractor is reinserted ifs needed to divide remaining adhesions under the direct vision.
-
At approximately the level of the third large vein along the lesser curvature, lymphatic tissue and vessels are mobilized and divided to expose the gastric wall. The right gastric vessels are preserved if possible. Starting from the lesser curvature of the stomach, several stapler loads of the linear cutting stapler are sequentially fired towards the fundus of the stomach, thus creating a 4–5 cm wide gastric conduit and ensuring a 5 cm margin distal to the tumor. Depending on the thickness of the stomach, medium purple or thick black (alternatively blue or green, depending on manufacturer) loads are used.
-
The gastric conduit stapler line is then oversewn with a running 4-0 (KP and JK) or 3-0 PDS (EA).
-
Illuminated transhiatal retractor is inserted into esophageal hiatus to inspect mediastinum and assure hemostasis.
-
An Saratoga sump drain is advanced from the cervical incision through the chest into the abdomen. A Penrose drain is sewn to the anterior wall of the gastric tube (in the area of expected gastrotomy for the anastomosis) and tied over the sump drain.
-
The gastric conduit is gently delivered through the mediastinum into the neck without torsion.
-
The right chest tube is taken of suction during conduit delivery. Left chest tube placement is optional if the left pleural cavity was not violated.
Cervical anastomosis (for cervical anastomosis operative images see Cervical gastroesophageal anastomosis page)
-
Cervical anastomosis is performed using the Modified Collard technique as described by Orringer.
-
The site of the gastrotomy is marked.
-
In very rare cases of a long available gastric tube length, its tipcan be shortened (as it is prone to ischemia) within the cervical incision with the linear cutting stapler to avoid its intrathoracic tortuosity before starting the anastomosis.
-
Packing the corner of a sponge under the gastric tip into the mediastinum at this point decreases slippage of the stomach into the mediastinum until the anastomosis is complete. The esophagus is aligned over the gastric tip.
-
The staple line of the esophagus is sharply removed. The NG tube is advanced out of the esophagus to help retract and align the esophagus for the anastomosis (alternatively pulled back proximally into the esophagus).
-
A gastrotomy is performed 3 cm distal to the tip of the staple line. Interrupted, full-thickness sutures are placed to align the midpoints posteriorly and anteriorly on the gastric conduit and esophagus for the anastomosis.
-
A 45 mm long linear cutting stapler is placed into the cervical esophagus and gastric conduit to create the posterior wall of the anastomosis.
-
Before the stapler is fired, additional seromuscular stitches are used to secure the tip of the conduit to the esophagus on each side proximally. The full thickness sutures for the running inner layer are placed into opposite corners at this point as well.
-
The stapler is fired to create the back wall of the anastomosis.
-
An NG tube is advanced through the anastomosis under direct visualization and positioned near the pylorus. This is confirmed by palpation through the abdomen at the end of the case before the NG is firmly secured at the nose.
-
The anterior aspect of the anastomosis is then completed with a full-thickness, running inner full-thickness layer of 3-0 or 4-0 PDS sutures run from opposite corners and tied in the middle.
-
An interrupted seromuscular layer of or 4-0 silk (KP, JK) or 3-0 Vicryl (EA) are used for the outer layer.
-
The supporting gastric conduit sponge can be removed at this point to allow anastomosis retract behind the manubrium.
-
A 10 Fr JP (KP, EA) or Penrose (JK) is placed by the anastomosis and directed into the superior mediastinum along the conduit.
-
The platysma is loosely approximated to the sternocleidomastoid muscle with a three or four interrupted Vicryl sutures. The skin is closed with running 4-0 Nylon.
Completion of the abdominal phase
-
The site for the feeding jejunostomy is located 20 cm distal to the duodenojejunal junction (commonly and incorrectly referred to as "ligament of Treitz")
-
16 Fr feeding Witzel or Stamm (JK) or jejunostomy (KP, EA) is placed. (See page Witzel jejunostomy).
-
Hemostasis in the abdomen is assured. Sponge counts are confirmed.
-
The fascia is closed with a #1 running absorbable suture.
-
The skin is closed with subcuticular sutures. Surgical dressing or skin adhesive is used to cover incision.
-
NG is flushed to confirm its patency and secured to nose with tape or a magnetic nasal bridle.
POSTOPERATIVE CARE
General
-
The patient is extubated in the operating room and admitted to a general care surgical ward.
-
Pain control provided via epidural catheter limiting systemic narcotic analgesics.
-
CXR is obtained immediately postoperatively. Daily CXR are not needed unless a change was made in the chest tube management or patient’s condition changes.
-
Isotonic fluids are used to maintain euvolemic balance, with close attention to in-and-out (I/O) fluid measurements.
-
MAP goal is >65 (to maintain perfusion of the conduit)
-
Perform urine output checks every 4 h. Consider removing indwelling urinary catheter after post-operative day 3 if patient is at low risk for having benign prostatic hyperplasia (BPH). Otherwise urinary catheter is kept while epidural is in place.
-
The head of bed is maintained at 45o to reduce the risk of aspiration.
-
NG tube to low (<80 mm Hg) continuous suction: Every 4 hours the drainage channel is flushed with 20 cc of fluid and the air channel is flushed with 20 cc of air to assure proper function of the NG tube. Functioning NG tube serves two goals:
-
After esophagectomy patients are prone to aspiration (due to lack of gastric storage capacity, absence of the lower esophageal sphincter (LES), and nonfunctional pylorus).
-
Thus the functioning NG is aimed to fully decompress the gastric conduit, which is especially important during postoperative ileus.
-
Additionally, the tip of the gastric conduit relies on the blood supply flow from the right gastroepiploic vessels through the intramural collaterals. Overdistention of the conduit may lead to low flow state in the collaterals, and hence ischemia of the anastomosis and decompression.
-
-
Chest tubes are maintained on suction at –20 cm H2O for 2-3 days (off suction for ambulation) to facilitate maximal lung expansion (for simplicity of use we prefer dry chamber).
-
Aggressive pulmonary care.
-
Ambulation at least four times daily is recommended.
-
Incentive spirometer every our hour.
-
Vibrating Positive Expiratory Pressure therapy every our hour.
-
Scheduled bronchodilators every 4 hours
-
Scheduled chest physical therapy with the use of manual Pneumatic Percussor on both lung fields every 4 hours while awake. Manual percussion even of the operative side is well tolerated by patients and may be more effective than a built in percussion function of hospital beds.
-
Use mucolytics (Acetylcysteine) inhalers as needed
-
-
Postoperative prophylactic antibiotics are not indicated.
-
Prophylactic heparin may be administered on POD 1.
Specifics
-
POD 1, 2—Pay attention to hemodynamics, I/O, electrolytes, pulmonary care, pain control, ambulation.
-
POD 3—Start tube feedings and advance to goal as tolerated.
-
POD 3—Start enteral route home medications
-
POD 4—NG and chest tubes are removed if there is no chyle leak while tube feedings at goal.
-
POD 5—Esophagram (water- soluble contrast followed by thin Barium) or JP drain fluid amylase.
-
JP removed if swallow is negative or amylase level is low.
-
Neck incision Nylon suture removed
-
Discharge with instructions to stay nil per os (NPO)
-
OUTPATIENT FOLLOW-UP
-
Close telephone follow-up by thoracic nurses.
-
Follow up in clinic in 2 weeks with chest radiogram (CXR)
-
Patient remains NPO for 3 weeks after esophagectomy.
-
If the patient develops a cervical wound infection (with or without obvious anastomotic leak), the incision is opened and packed. Such patients are at high risk for developing anastomotic stricture and should undergo endoscopy within 2-4weeks, with dilation if needed.
-
Patients are started on a clear liquid diet and, with increments of every 3 days, advanced to soft mechanical and then regular diet under close telephone follow-up by thoracic nurses.
-
Follow up with PET computed tomography every 6 months for 3 years and then as clinically indicated as per National Comprehensive Cancer Network guidelines (https://www.nccn.org/professionals/physician_gls/default.aspx).
______________________________________________________________________________________