
Four years ago, UI scientist Long-Sheng Song, MD, made an unexpected discovery, which he thought might form the basis for a new gene therapy treatment for heart failure. In his latest study, he shows that the approach does work in mice, protecting the animals from heart failure due to cardiac stress.
Heart failure occurs when the heart muscle becomes weak and damaged and cannot pump blood efficiently round the body. It is a leading cause of death and illness worldwide, affecting more than 26 million people. Cardiac stress, from high blood pressure, blocked arteries, or heart attack, is one cause of heart failure.
In the earlier study, Song and his team found that during cardiac stress, a protein known as junctophilin-2 (JP2) is cleaved into two pieces and one of those fragments (JP2NT) can turn off the expression of certain damaging genes in heart cells. He speculated that harnessing this self-protecting mechanism might lead to a new treatment for heart failure.
In the new study, published recently in the journal Circulation Research, Song’s team shows that delivering the JP2NT fragment to heart cells using adeno-associated virus mediated gene delivery method can limit the development of heart failure when it is given either before or after the onset of cardiac stress.
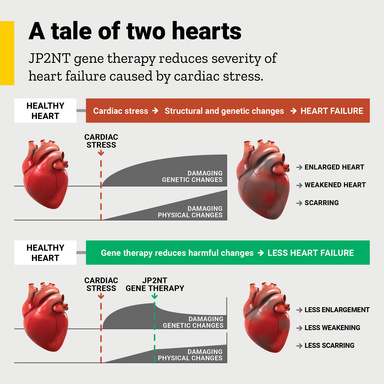
“The fact that this gene therapy approach was able to slow the development of heart failure even after cardiac stress had started to damage the heart muscle, suggests that JP2NT gene therapy holds great promise as a novel treatment for heart failure,” says Song, UI professor of internal medicine-cardiovascular medicine and a member of the François M. Abboud Cardiovascular Research Center and the Fraternal Order of Eagles Diabetes Research Center at the UI.
The researchers plan to further test this gene therapy approach in large animal models and, if the results are promising, pursue a clinical trial in patients with heart failure.
The research in Song’s group was primarily conducted by Jinxi Wang, PhD, a postdoctoral fellow in the lab. Other contributors include Qian Shi, Yihui Wang, Logan Dawson, Grace Ciampa, Weiyang Zhao, Guangqin Zhang, Biyi Chen, Robert Weiss, Chad Grueter, and Duane Hall.
The study was supported by funding from the Albaghdadi Family Medical Foundation, American Heart Association, National Heart, Lung and Blood Institutes, and the Department of Veterans Affairs of the United States.